EfavirenzReverse transcriptase inhibitor CAS# 154598-52-4 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154598-52-4 | SDF | Download SDF |
| PubChem ID | 64139 | Appearance | Powder |
| Formula | C14H9ClF3NO2 | M.Wt | 315.68 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DMP 266; EFV; L-743726 | ||
| Solubility | DMSO : ≥ 38 mg/mL (120.38 mM) *"≥" means soluble, but saturation unknown. | ||
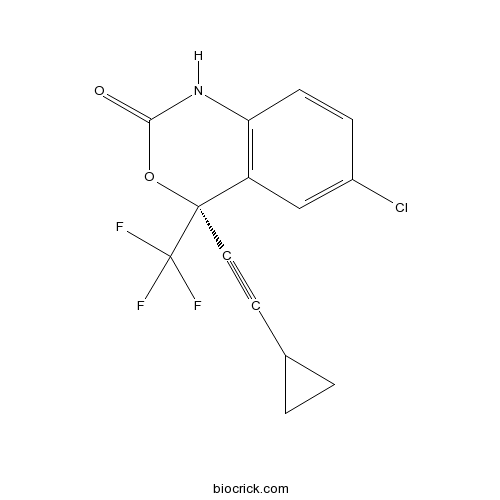
| Chemical Name | (4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1H-3,1-benzoxazin-2-one | ||
| SMILES | C1CC1C#CC2(C3=C(C=CC(=C3)Cl)NC(=O)O2)C(F)(F)F | ||
| Standard InChIKey | XPOQHMRABVBWPR-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Efavirenz is a potent inhibitor of the wild-type HIV-1 RT (Ki=2.93 nM) and exhibits IC95 of 1.5 nM for the inhibition of HIV-1 replicative spread in cell culture.In Vitro:Efavirenz (L-743726) is found to be capable of inhibiting, with 95% inhibitory concentrations of ≤ 1.5μM, a panel of nonnucleoside reverse transcriptase (RT) inhibitors (NNRTIs)-resistant mutant viruses, each of which expresses a single RT amino acid substitution. Efavirenz is also tested for its activity against a variety of polymerase enzymes and is found to be inactive (IC50>300μM). Efavirenz effectively inhibits several wild-type T-lymphoid cell line-adapted variants. Identical activity (IC95, 1.5 to 3.0 nM) is seen with wild-type primary isolates of the virus in both primary lymphoid and monocytoid cell cultures. Efavirenz also effectively inhibits HIV-1 variants that expressed RT amino acid substitutions which confer the loss of susceptibility to other NNRTIs. For purposes of comparison[1]. Efavirenz is a non-nucleoside analog reverse transcriptase inhibitor (NNRTI) with IC50 of 60 nM[2]. Efavirenz inhibits synthesis using an RNA PPT-primed substrate with an IC50 of 17 nM[3].In Vivo:After i.v. administration, Efavirenz (L-743726) is cleared rapidly from rats, but it is cleared considerably more slowly from monkeys. The large volume of distribution (two to four times the amount of body water) in both species indicates extensive tissue binding. The oral bioavailability in rats is 16%. In monkeys, the half-life of Efavirenz after administration of a 1 mg/kg i.v. dose exceeded 2.5 h. Efavirenz is well absorbed orally. Administration to monkeys of oral doses as fine suspensions in 0.5% aqueous methylcellulose yields consistently high levels in plasma. A 2.0 mg/kg dose produces peak levels of 0.5μM at approximately 3.0 h. The absolute bioavailability is estimated to be 42%. A 10 mg/kg dose yields a peak level in plasma of 3.22 μM. A 10 mg/kg oral dose given to a single chimpanzee gave concentrations in plasma of 4.12, 2.95, and 2.69 μM at 2, 8, and 24 h after dosing, respectively[1]. References: | |||||

Efavirenz Dilution Calculator

Efavirenz Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1678 mL | 15.8388 mL | 31.6776 mL | 63.3553 mL | 79.1941 mL |
| 5 mM | 0.6336 mL | 3.1678 mL | 6.3355 mL | 12.6711 mL | 15.8388 mL |
| 10 mM | 0.3168 mL | 1.5839 mL | 3.1678 mL | 6.3355 mL | 7.9194 mL |
| 50 mM | 0.0634 mL | 0.3168 mL | 0.6336 mL | 1.2671 mL | 1.5839 mL |
| 100 mM | 0.0317 mL | 0.1584 mL | 0.3168 mL | 0.6336 mL | 0.7919 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Efavirenz is a highly potent inhibitor of human immunodeficiency virus type 1 reverse transcriptase with Ki value of 2.93nM [1].
Efavirenz is an antiretroviral drug approved by the FDA in 1998. It is usually used in a combination therapy with other antiretroviral drugs. Efavirenz is a potent inhibitor of both wild-type HIV-1 RT and HIV-1 variants which express series of NNRTI resistance-associated amino acid substitutions. The Ki value of efavirenz against the purified wild-type HIV-1 RT is 2.93nM. For the mutants A98G, L100I, K101G and K103N, the Ki values are 3.85nM, 17.13nM, 7.27nM and 17.6nM, respectively. Efavirenz is also a selective inhibitor of HIV-1 RT. It shows no inhibitory activity against a variety of polymerase enzymes including avian myeloblastosis virus RT, Moloney murine leukemia virus RT, human DNA polymerases and Escherichia coli RNA polymerase. In the acute infection assay, efavirenz also exerts potency with both wild-type HIV-1 RT (IC95s ranging from 1.5nM-3nM) and mutant HIV-1 RT [1, 2].
References:
[1] Young S D, Britcher S F, Tran L O, et al. L-743, 726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrobial agents and chemotherapy, 1995, 39(12): 2602-2605.
[2] de Béthune M P. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years (1989–2009). Antiviral research, 2010, 85(1): 75-90.
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- 3-Methoxymollugin
Catalog No.:BCN7164
CAS No.:154706-44-2
- 2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol
Catalog No.:BCN1555
CAS No.:154801-30-6
- Cimiside E
Catalog No.:BCN7951
CAS No.:154822-57-8
- 4-Acetoxycinnamic acid
Catalog No.:BCN5026
CAS No.:15486-19-8
- Eleutheroside C
Catalog No.:BCN1690
CAS No.:15486-24-5
- 3,5-Dihydroxy-4',7-dimethoxyflavone
Catalog No.:BCN1691
CAS No.:15486-33-6
- Kaempferol 3,7,4'-trimethylether
Catalog No.:BCN4087
CAS No.:15486-34-7
- LL 37
Catalog No.:BCC8027
CAS No.:154947-66-7
- BAY-u 9773
Catalog No.:BCC7576
CAS No.:154978-38-8
- Erysenegalensein E
Catalog No.:BCN3979
CAS No.:154992-17-3
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
Association of IL10, IL4, IFNG, and CTLA4 Gene Polymorphisms with Efavirenz Hypersensitivity Reaction in Patients Infected with Human Immunodeficiency Virus.[Pubmed:28250252]
Jpn J Infect Dis. 2017 Jul 24;70(4):430-436.
We evaluated interleukin-10 (IL10) 592 C/A, IL4589 C/T, interferon gamma (IFNG)874 A/T, cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)49 A/G gene polymorphisms associated with Efavirenz hypersensitivity reaction. A total of 63 human immunodeficiency virus-positive patients under treatment at a public hospital were included in the study, of whom 21 presented with Efavirenz hypersensitivity. Patients who presented with Efavirenz hypersensitivity reaction showed a higher frequency of the IL10 592A allele than the controls (p0.028). The allele A was associated with increased risk of Efavirenz hypersensitivity (odds ratio2.40). In case of IL4, a significant difference in the frequency of the IL4 589 (C/T) polymorphism was not observed between patients and controls. A significant inverse correlation was observed when comparing the CTLA449A/G and IL4 589 C/T polymorphisms (r0.650, p0.001); that is, the CTLA4 +49GG genotype, involved with the lowest capacity of inhibition, was inversely correlated IL4589TT genotype, which induces high production of IL-4. With respect to the CTLA449A/G and IFNG874T/A gene polymorphisms, significant differences in allele and genotype frequencies were not observed between the groups. Therefore, our data suggest that polymorphisms in regulatory regions of cytokine genes could modulate an individual's susceptibility to Efavirenz hypersensitivity reaction.
Switching from efavirenz, emtricitabine, and tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study.[Pubmed:28259776]
Lancet HIV. 2017 May;4(5):e205-e213.
BACKGROUND: Tenofovir alafenamide is a prodrug that reduces tenofovir plasma concentrations by 90% compared with tenofovir disoproxil fumarate, thereby decreasing bone and renal risks. The coformulation of rilpivirine, emtricitabine, and tenofovir alafenamide has recently been approved, and we aimed to investigate the efficacy, safety, and tolerability of switching to this regimen compared with remaining on coformulated Efavirenz, emtricitabine, and tenofovir disoproxil fumarate. METHODS: In this randomised, double-blind, placebo-controlled, non-inferiority trial, HIV-1-infected adults were enrolled at 120 hospitals and outpatient clinics in eight countries in North America and Europe. Participants were virally suppressed (HIV-1 RNA <50 copies per mL) on Efavirenz, emtricitabine, and tenofovir disoproxil fumarate for at least 6 months before enrolment and had creatinine clearance of at least 50 mL/min. Participants were randomly assigned (1:1) to receive a single-tablet regimen of rilpivirine (25 mg), emtricitabine (200 mg), and tenofovir alafenamide (25 mg) or to continue a single-tablet regimen of Efavirenz (600 mg), emtricitabine (200 mg), and tenofovir disoproxil fumarate (300 mg), with matching placebo. Investigators, participants, study staff, and those assessing outcomes were masked to treatment group. The primary endpoint was the proportion of participants with plasma HIV-1 RNA of less than 50 copies per mL at week 48 (assessed by the US Food and Drug Administration snapshot algorithm), with a prespecified non-inferiority margin of 8%. This study was registered with ClinicalTrials.gov, number NCT02345226. FINDINGS: Between Jan 26, 2015, and Aug 27, 2015, 875 participants were randomly assigned and treated (438 with rilpivirine, emtricitabine, and tenofovir alafenamide and 437 with Efavirenz, emtricitabine, tenofovir disoproxil fumarate). Viral suppression at week 48 was maintained in 394 (90%) of 438 participants assigned to the tenofovir alafenamide regimen and 402 (92%) of 437 assigned to the tenofovir disoproxil fumarate regimen (difference -2.0%, 95.001% CI -5.9 to 1.8), demonstrating non-inferiority. 56 (13%) of 438 in participants in the rilpivirine, emtricitabine, and tenofovir alafenamide group experienced treatment-related adverse events compared with 45 (10%) of 437 in the Efavirenz, emtricitabine, and tenofovir disoproxil fumarate group. INTERPRETATION: Switching to rilpivirine, emtricitabine, and tenofovir alafenamide from Efavirenz, emtricitabine, and tenofovir disoproxil fumarate was non-inferior in maintaining viral suppression and was well tolerated at 48 weeks. These findings support guidelines recommending tenofovir alafenamide-based regimens, including coformulation with rilpivirine and emtricitabine, as initial and ongoing treatment for HIV-1 infection. FUNDING: Gilead Sciences.
CYP2B6 genotype-directed dosing is required for optimal efavirenz exposure in children 3-36 months with HIV infection.[Pubmed:28323755]
AIDS. 2017 May 15;31(8):1129-1136.
OBJECTIVES: To determine safety-specific, efficacy-specific and genotypic-specific dose requirements of Efavirenz (EFV) in children aged 3 to less than 36 months with HIV infection. DESIGN: IMPAACT P1070 was a 24-week prospective cohort trial of EFV (as open capsules) and two nucleoside reverse transcriptase inhibitors in children with HIV infection 3 to less than 36 months without tuberculosis (Cohort 1). METHODS: CYP2B6 G516T genotype was determined, and intensive pharmacokinetics was performed at week 2. EFV dose was adjusted if outside the target area under the curve (AUC) 35-180 mug*h/ml. Pharmacokinetic and CYP2B6 G516T genotype data were used to model EFV exposures based on Food and Drug Administration (FDA)-approved doses. RESULTS: Forty-seven participants, median age 19 months, initiated the study regimen with 24 weeks median follow-up; 38 516GG/GT and 9 516TT genotypes. Initially, median EFV AUC was higher in 516TT vs. 516GG/GT (median 490 vs. 107 mug*h/ml; P = 0.0001) with all 516TT above AUC target. Following an amendment that reduced the 516TT EFV dose by 75%, pharmacokinetic modeling predicted that 83% of participants met the AUC target (31/38 516GG/GT, 8/9 516TT). In contrast, modeling using P1070 data predicted that FDA-approved doses would produce subtherapeutic AUCs in almost one-third of participants with 516GG/GT and excessive AUCs in more than 50% with 516TT genotypes. CONCLUSION: CYP2B6 G516T genotype strongly influences EFV exposures in this age group. Genotype-directed dosing yields therapeutic EFV concentrations and appears to outperform other dosing approaches.
Does efavirenz replacement improve neurological function in treated HIV infection?[Pubmed:28247479]
HIV Med. 2017 Oct;18(9):690-695.
OBJECTIVES: The contribution of specific antiretroviral drugs to cognitive function in HIV-infected people remains poorly understood. Efavirenz (EFV) may plausibly cause cognitive impairment. The objective of this study was therefore to determine whether chronic EFV therapy is a modifier of neurocognitive and neurometabolic function in the setting of suppressive highly active antiretroviral therapy. METHODS: We performed an open-label phase IV controlled trial. Adult subjects who were stable on suppressive EFV therapy for at least 6 months were switched to ritonavir-boosted lopinavir (LPV/r) with no change in the nucleoside reverse transcriptase inhibitor (NRTI) backbone. The following parameters were assessed before and 10 weeks after therapy switch: cognitive function (by CogState((R)) computerized battery); brain metabolites (by proton magnetic resonance spectroscopy); brain activity [by attentional processing task-based functional magnetic resonance imaging]; and sleep quantity and quality [by sleep diary, Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale]. RESULTS: Sixteen subjects completed the study. Despite most subjects (81%) self-reporting memory problems at baseline, cognitive function, brain metabolites, and brain activity showed no change at 10 weeks after switch. Sleep quality improved on switch off EFV [mean PSQI (standard deviation): EFV, 8.5 (6.5); LPV/r, 5.8 (5.5); mean difference -0.4; 95% confidence interval -6.0 to -0.7]. CONCLUSIONS: This is the first study to assess the effects of chronic EFV therapy on neurological function in a controlled setting. We conclude that EFV withdrawal is unlikely to result in significant modification of neurocognitive function in otherwise stable HIV-infected people.


