Fmoc-Arg(Pbf)-OHCAS# 154445-77-9 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154445-77-9 | SDF | Download SDF |
| PubChem ID | 11354259 | Appearance | Powder |
| Formula | C34H40N4O7S | M.Wt | 648.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
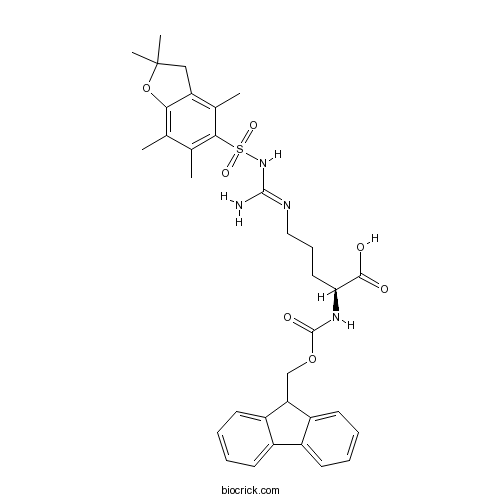
| Chemical Name | (2S)-5-[[amino-[(2,2,4,6,7-pentamethyl-3H-1-benzofuran-5-yl)sulfonylamino]methylidene]amino]-2-(9H-fluoren-9-ylmethoxycarbonylamino)pentanoic acid | ||
| SMILES | CC1=C2C(=C(C(=C1C)S(=O)(=O)NC(=NCCCC(C(=O)O)NC(=O)OCC3C4=CC=CC=C4C5=CC=CC=C35)N)C)CC(O2)(C)C | ||
| Standard InChIKey | HNICLNKVURBTKV-NDEPHWFRSA-N | ||
| Standard InChI | InChI=1S/C34H40N4O7S/c1-19-20(2)30(21(3)26-17-34(4,5)45-29(19)26)46(42,43)38-32(35)36-16-10-15-28(31(39)40)37-33(41)44-18-27-24-13-8-6-11-22(24)23-12-7-9-14-25(23)27/h6-9,11-14,27-28H,10,15-18H2,1-5H3,(H,37,41)(H,39,40)(H3,35,36,38)/t28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Arg(Pbf)-OH Dilution Calculator

Fmoc-Arg(Pbf)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5413 mL | 7.7065 mL | 15.4131 mL | 30.8261 mL | 38.5327 mL |
| 5 mM | 0.3083 mL | 1.5413 mL | 3.0826 mL | 6.1652 mL | 7.7065 mL |
| 10 mM | 0.1541 mL | 0.7707 mL | 1.5413 mL | 3.0826 mL | 3.8533 mL |
| 50 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6165 mL | 0.7707 mL |
| 100 mM | 0.0154 mL | 0.0771 mL | 0.1541 mL | 0.3083 mL | 0.3853 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Arg(Pbf)-OH
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- 3-Methoxymollugin
Catalog No.:BCN7164
CAS No.:154706-44-2
- 2-(2'-Hydroxytetracosanoylamino)-octadecane-1,3,4-triol
Catalog No.:BCN1555
CAS No.:154801-30-6
A 'conovenomic' analysis of the milked venom from the mollusk-hunting cone snail Conus textile--the pharmacological importance of post-translational modifications.[Pubmed:24055806]
Peptides. 2013 Nov;49:145-58.
Cone snail venoms provide a largely untapped source of novel peptide drug leads. To enhance the discovery phase, a detailed comparative proteomic analysis was undertaken on milked venom from the mollusk-hunting cone snail, Conus textile, from three different geographic locations (Hawai'i, American Samoa and Australia's Great Barrier Reef). A novel milked venom conopeptide rich in post-translational modifications was discovered, characterized and named alpha-conotoxin TxIC. We assign this conopeptide to the 4/7 alpha-conotoxin family based on the peptide's sequence homology and cDNA pre-propeptide alignment. Pharmacologically, alpha-conotoxin TxIC demonstrates minimal activity on human acetylcholine receptor models (100 muM, <5% inhibition), compared to its high paralytic potency in invertebrates, PD50 = 34.2 nMol kg(-1). The non-post-translationally modified form, [Pro](2,8)[Glu](16)alpha-conotoxin TxIC, demonstrates differential selectivity for the alpha3beta2 isoform of the nicotinic acetylcholine receptor with maximal inhibition of 96% and an observed IC50 of 5.4 +/- 0.5 muM. Interestingly its comparative PD50 (3.6 muMol kg(-1)) in invertebrates was ~100 fold more than that of the native peptide. Differentiating alpha-conotoxin TxIC from other alpha-conotoxins is the high degree of post-translational modification (44% of residues). This includes the incorporation of gamma-carboxyglutamic acid, two moieties of 4-trans hydroxyproline, two disulfide bond linkages, and C-terminal amidation. These findings expand upon the known chemical diversity of alpha-conotoxins and illustrate a potential driver of toxin phyla-selectivity within Conus.
Identification of Fmoc-beta-Ala-OH and Fmoc-beta-Ala-amino acid-OH as new impurities in Fmoc-protected amino acid derivatives.[Pubmed:15686539]
J Pept Res. 2005 Jan;65(1):90-7.
During the manufacture of a proprietary peptide drug substance a new impurity appeared unexpectedly. Investigation of its chemical structure established the impurity as a beta-Ala insertion mutant of the mother peptide. The source of the beta-Ala was identified as contamination of the Fmoc-Ala-OH raw material with Fmoc-beta-Ala-Ala-OH. Further studies also demonstrated the presence of beta-Ala in other Fmoc-amino acids, particularly in Fmoc-Arg(Pbf)-OH. In this case, it was due to the presence of both Fmoc-beta-Ala-OH and Fmoc-beta-Ala-Arg(Pbf)-OH. It is concluded that beta-Ala contamination of Fmoc-amino acid derivatives is a general and hitherto unrecognized problem to suppliers of Fmoc-amino acid derivatives. The beta-Ala is often present as Fmoc-beta-Ala-OH and/or as a dipeptide, Fmoc-beta-Ala-amino acid-OH. In collaboration with the suppliers, new specifications were introduced, recognizing the presence of beta-Ala-related impurities in the raw materials and limiting them to acceptable levels. The implementation of these measures has essentially eliminated beta-Ala contamination as a problem in the manufacture of the drug substance.


