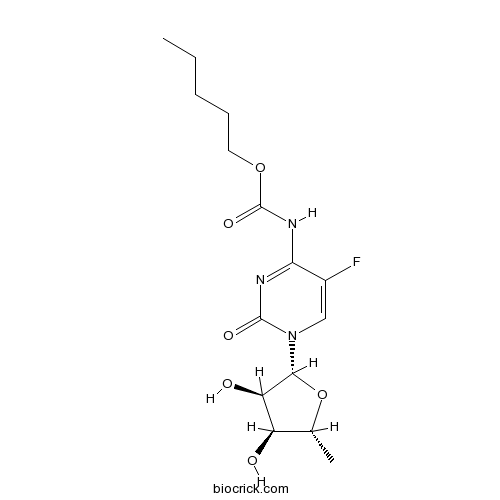
CapecitabineCAS# 154361-50-9 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154361-50-9 | SDF | Download SDF |
| PubChem ID | 60953 | Appearance | Powder |
| Formula | C15H22FN3O6 | M.Wt | 359.35 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Ro 09-1978 | ||
| Solubility | DMSO : 100 mg/mL (278.28 mM; Need ultrasonic) H2O : ≥ 33.33 mg/mL (92.75 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | pentyl N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]carbamate | ||
| SMILES | CCCCCOC(=O)NC1=NC(=O)N(C=C1F)C2C(C(C(O2)C)O)O | ||
| Standard InChIKey | GAGWJHPBXLXJQN-UORFTKCHSA-N | ||
| Standard InChI | InChI=1S/C15H22FN3O6/c1-3-4-5-6-24-15(23)18-12-9(16)7-19(14(22)17-12)13-11(21)10(20)8(2)25-13/h7-8,10-11,13,20-21H,3-6H2,1-2H3,(H,17,18,22,23)/t8-,10-,11-,13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Capecitabine-induced leukocytoclastic vasculitis under neoadjuvant chemotherapy for locally advanced colorectal cancer. |
| In vivo | Capecitabine-induced leukocytoclastic vasculitis under neoadjuvant chemotherapy for locally advanced colorectal cancer.[Pubmed: 26029464]J Gastrointest Oncol. 2015 Jun;6(3):E40-3.We describe a case of Capecitabine-induced leukocytoclastic vasculitis in a patient with locally advanced rectal cancer under curative neoadjuvant concurrent chemoradiation using Capecitabine. Observational study of adjuvant therapy with capecitabine in colon cancer.[Pubmed: 25651480]Curr Med Res Opin. 2015 Apr;31(4):731-41.This observational study was conducted to document the safety of Capecitabine-based adjuvant therapy in patients with resected colon cancer under routine clinical conditions. Concomitant capecitabine with hepatic delivery of drug eluting beads in metastatic colorectal cancer.[Pubmed: 25503155]Anticancer Res. 2014 Dec;34(12):7239-45. Effectiveness and toxicity of transcatheter arterial injection of irinotecan-eluting beads (DEBIRI) with and without concurrent Capecitabine in pre-treated patients with metastatic colorectal cancer (CRC). |

Capecitabine Dilution Calculator

Capecitabine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7828 mL | 13.914 mL | 27.828 mL | 55.656 mL | 69.5701 mL |
| 5 mM | 0.5566 mL | 2.7828 mL | 5.5656 mL | 11.1312 mL | 13.914 mL |
| 10 mM | 0.2783 mL | 1.3914 mL | 2.7828 mL | 5.5656 mL | 6.957 mL |
| 50 mM | 0.0557 mL | 0.2783 mL | 0.5566 mL | 1.1131 mL | 1.3914 mL |
| 100 mM | 0.0278 mL | 0.1391 mL | 0.2783 mL | 0.5566 mL | 0.6957 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Prodrug of 5-Fluorouracil (5-FU). Selectively activated in tumor cells by thymidine phosphorylase; inhibits DNA synthesis upon conversion to 5-FU. Orally available.
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
Capecitabine-induced leukocytoclastic vasculitis under neoadjuvant chemotherapy for locally advanced colorectal cancer.[Pubmed:26029464]
J Gastrointest Oncol. 2015 Jun;6(3):E40-3.
We describe a case of Capecitabine-induced leukocytoclastic vasculitis in a patient with locally advanced rectal cancer under curative neoadjuvant concurrent chemoradiation using Capecitabine. After 5 days of the initiation of Capecitabine the patient developed a pruritic maculopapular rash in her extremities consistent with vasculitis which was confirmed on skin biopsy without any signs of systemic involvement. Capecitabine was held and the rash was treated with topical steroids with complete resolution of both rash and pruritus. Due to a lack of other alternative chemotherapeutic options and the cutaneous-only involvement of vasculitis, the Capecitabine was re-introduced. Two days later, the patient developed an identical maculopapular rash with a similar distribution. Prednisone was initiated while the Capecitabine was continued with complete resolution of the rash. The patient successfully completed her curative neoadjuvant chemoradiation therapy treatment without the need to permanently discontinue the Capecitabine.
Genomic alterations in DNA repair and chromatin remodeling genes in estrogen receptor-positive metastatic breast cancer patients with exceptional responses to capecitabine.[Pubmed:25871911]
Cancer Med. 2015 Aug;4(8):1289-93.
We analyzed the genomic and phosphoproteomic profiles of breast cancer tissue obtained from six patients with estrogen receptor (ER)-positive, HER2-negative metastatic breast cancer who had highly durable (>/= 5 years) and, in some cases, ongoing clinical responses with Capecitabine. Formalin-fixed, paraffin-embedded tissue samples from patients' primary (n = 4) or metastatic (n = 2) breast cancers were utilized for targeted next-generation sequencing and reversed phase protein microarray. Two patients received Capecitabine monotherapy. Four patients received Capecitabine in combination with paclitaxel; three of these continued single-agent Capecitabine after stopping paclitaxel. Capecitabine was discontinued for progressive disease after a mean of 66 months in four patients (range 54-86 months), and two patients remain on therapy, having received Capecitabine for >91 months and >122 months, respectively. Three patients' cancers (50%) had likely functional alterations in DNA repair and chromatin remodeling genes, while three other patients' cancers had variants of unknown significance in these pathways. Mutations in PIK3CA, amplifications of FGFR1 or ZNF703, or phosphorylation of HER family receptors and their downstream proteins did not preclude exceptional responses to Capecitabine. None of the patients' tumors harbored TP53 or PTEN mutations. Four of the patients had breast cancer tissue available for PTEN immunohistochemistry, and all four patients' cancers were positive for PTEN. These surprising findings in a group of phenotypically similar patients with ER-positive, endocrine therapy-pretreated, HER2-negative metastases, are supported by preclinical data showing that sensitivity to 5-fluorouracil is enhanced by deficiencies in chromatin remodeling and homologous recombination genes. Our findings suggest that mutations that inactivate homologous recombination and/or chromatin remodeling genes within ER-positive, HER2-negative breast cancers may predict for highly durable responses to Capecitabine.
Concomitant capecitabine with hepatic delivery of drug eluting beads in metastatic colorectal cancer.[Pubmed:25503155]
Anticancer Res. 2014 Dec;34(12):7239-45.
BACKGROUND: Effectiveness and toxicity of transcatheter arterial injection of irinotecan-eluting beads (DEBIRI) with and without concurrent Capecitabine in pre-treated patients with metastatic colorectal cancer (CRC). PATIENTS AND METHODS: An Institutional Review Board-approved, multi-institutional registry from 5/2008 to 8/2013 was reviewed. Patients who received DEBIRI with (X-DEBIRI) or without (DEBIRI) Capecitabine were compared. RESULTS: Twenty-two X-DEBIRI and 149 DEBIRI patients were compared. There was no difference in the two groups with regards to adverse events (p=0.56). During a 3- and 6-month evaluation, the disease control rate (DCR) was similar in both groups. During the 12-month evaluation, there was better DCR in the X-DEBIRI group (p=0.03). Median survival was 13 months in the DEBIRI group and 22 months in the X-DEBIRI group (log-rank test, p=0.217). CONCLUSION: There is no additional toxicity when adding Capecitabine with DEBIRI. Concurrent Capecitabine may offer more durable disease control rate compared to DEBIRI-alone. Survival benefit with concurrent Capecitabine was not statistically significant but there may be a trend towards improved survival.
Observational study of adjuvant therapy with capecitabine in colon cancer.[Pubmed:25651480]
Curr Med Res Opin. 2015 Apr;31(4):731-41.
OBJECTIVE: This observational study was conducted to document the safety of Capecitabine-based adjuvant therapy in patients with resected colon cancer under routine clinical conditions. RESEARCH AND DESIGN METHODS: ML20431 was a prospective, multicenter, non-interventional, observational study. It was designed to answer five research questions relating to safety, dosage and administration, and discontinuation from Capecitabine-based adjuvant therapy. Patients were required to have R0 resected stage III colon cancer and have started treatment with Capecitabine-based adjuvant therapy based on a decision by the investigator. Patients were followed over an observation period of Capecitabine and formed the analysis population. Most patients had colon cancer (78.3%), followed by rectal cancer (16.4%). Most patients had stage III disease (69.3%); the remaining patients had stage II disease (30.7%). The most common all-grade adverse reactions were hand-foot syndrome (46.9%), diarrhea (34.4%), and hemoglobin decreases (31.5%). Grade 3/4 adverse reactions were infrequent (<4%). Serious adverse events were reported in 96 patients (6.5%). Six or more cycles of treatment were completed by 77.9% of patients. Approximately two-thirds of patients (67.3%) received Capecitabine monotherapy and the remainder (32.7%) received Capecitabine in combination with >/=1 drugs, most commonly oxaliplatin (460 cases). Discontinuation of Capecitabine was documented in 344 patients (23.2%). STUDY LIMITATIONS: no efficacy data were collected; the questionnaires for patients' expectations and satisfaction were not formally validated; and a few patients (<1.5%) had some retrospective data. CONCLUSIONS: The safety profile of Capecitabine-based adjuvant therapy in a broad patient population with colon cancer is similar to that previously documented in phase III clinical trials.
Metabolism of capecitabine, an oral fluorouracil prodrug: (19)F NMR studies in animal models and human urine.[Pubmed:12386128]
Drug Metab Dispos. 2002 Nov;30(11):1221-9.
Capecitabine (Xeloda; CAP) is a recently developed oral antineoplastic prodrug of 5-fluorouracil (5-FU) with enhanced tumor selectivity. Previous studies have shown that CAP activation follows a pathway with three enzymatic steps and two intermediary metabolites, 5'-deoxy-5-fluorocytidine (5'-DFCR) and 5'-deoxy-5-fluorouridine (5'-DFUR), to form 5-FU preferentially in tumor tissues. In the present work, we investigated all fluorinated compounds present in liver, bile, and perfusate medium of isolated perfused rat liver (IPRL) and in liver, plasma, kidneys, bile, and urine of healthy rats. Moreover, data obtained from rat urine were compared with those from mice and human urine. According to a low cytidine deaminase (3.5.4.5) activity in rats, 5'-DFCR was by far the main product in perfusate medium from IPRL and plasma and urine from rats. Liver and circulating 5'-DFCR in perfusate and plasma equilibrated at the same concentration value in the range 25 to 400 microM, which supports the involvement of es-type nucleoside transporter in the liver. 5'-DFUR and alpha-fluoro-beta-ureidopropionic acid (FUPA) + alpha-fluoro-beta-alanine (FBAL) were the main products in urine of mice, making up 23 to 30% of the administered dose versus 3 to 4% in rat. In human urine, FUPA + FBAL represented 50% of the administered dose, 5'-DFCR 10%, and 5'-DFUR 7%. Since fluorine-19 nuclear magnetic resonance spectroscopy gives an overview of all the fluorinated compounds present in a sample, we observed the following unreported metabolites of CAP: 1) 5-fluorocytosine and its hydroxylated metabolite, 5-fluoro-6-hydroxycytosine, 2) fluoride ion, 3) 2-fluoro-3-hydroxypropionic acid and fluoroacetate, and 4) a glucuroconjugate of 5'-DFCR.
The design and synthesis of a new tumor-selective fluoropyrimidine carbamate, capecitabine.[Pubmed:10976516]
Bioorg Med Chem. 2000 Jul;8(7):1697-706.
To identify an orally available fluoropyrimidine having efficacy and safety profiles greatly improved over those of parenteral 5-fluorouracil (5-FU: 1), we designed a 5-FU prodrug that would pass intact through the intestinal mucisa and be sequentially converted to 5-FU by enzymes that are highly expressed in the human liver and then in tumors. Among various N4-substituted 5'-deoxy-5-fluorocytidine derivatives, a series of N4-alkoxycarbonyl derivatives were hydrolyzed to 5'-deoxy-5-fluorocytidine (5'-DFCR: 8) specifically by carboxylesterase, which exists preferentially in the liver in humans and monkeys. Particularly, derivatives having an N4-alkoxylcarbonyl moiety with a C4-C6 alkyl chain were the most susceptible to the human carboxylesterase. Those were then converted to 5'-deoxy-5-fluorouridine (5'-DFUR: 4) by cytidine deaminase highly expressed in the liver and solid tumors and finally to 5-FU by thymidine phosphorylase (dThdPase) preferentially located in tumors. When administered orally to monkeys, a derivative having the N4-alkoxylcarbonyl moiety with a C5 alkyl chain (Capecitabine: 6) The highest AUC and Cmax for plasma 5'-DFUR. In tests with various human cancer xenograft models, Capecitabine was more efficacious at wider dose ranges than either 5-FU or 5'-DFUR and was significantly less toxic to the intestinal tract than the others in monkeys.


