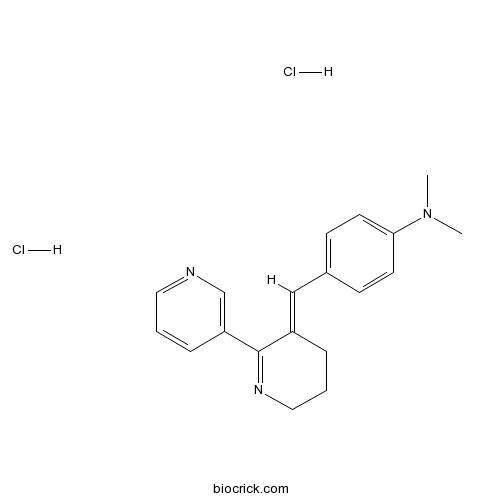
DMAB-anabaseine dihydrochlorideCAS# 154149-38-9 |
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- TFB-TBOA
Catalog No.:BCC5919
CAS No.:480439-73-4
- Dihydrokainic acid
Catalog No.:BCC6556
CAS No.:52497-36-6
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- LDN 212320
Catalog No.:BCC6361
CAS No.:894002-50-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154149-38-9 | SDF | Download SDF |
| PubChem ID | 16759167 | Appearance | Powder |
| Formula | C19H23Cl2N3 | M.Wt | 364.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 25 mM in DMSO | ||
| Chemical Name | N,N-dimethyl-4-[(E)-(6-pyridin-3-yl-3,4-dihydro-2H-pyridin-5-ylidene)methyl]aniline;dihydrochloride | ||
| SMILES | CN(C)C1=CC=C(C=C1)C=C2CCCN=C2C3=CN=CC=C3.Cl.Cl | ||
| Standard InChIKey | DHCNEWWWKHBASC-YNHPUKFJSA-N | ||
| Standard InChI | InChI=1S/C19H21N3.2ClH/c1-22(2)18-9-7-15(8-10-18)13-16-5-4-12-21-19(16)17-6-3-11-20-14-17;;/h3,6-11,13-14H,4-5,12H2,1-2H3;2*1H/b16-13+;; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Partial agonist at α7-containing neuronal nicotinic receptors and antagonist at α4β2 and other nicotinic receptors. Displays specific cognition-enhancing effects; improves long-term memory in rats. |

DMAB-anabaseine dihydrochloride Dilution Calculator

DMAB-anabaseine dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7448 mL | 13.7242 mL | 27.4484 mL | 54.8968 mL | 68.621 mL |
| 5 mM | 0.549 mL | 2.7448 mL | 5.4897 mL | 10.9794 mL | 13.7242 mL |
| 10 mM | 0.2745 mL | 1.3724 mL | 2.7448 mL | 5.4897 mL | 6.8621 mL |
| 50 mM | 0.0549 mL | 0.2745 mL | 0.549 mL | 1.0979 mL | 1.3724 mL |
| 100 mM | 0.0274 mL | 0.1372 mL | 0.2745 mL | 0.549 mL | 0.6862 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Berberrubine
Catalog No.:BCN2651
CAS No.:15401-69-1
- Ipecoside
Catalog No.:BCN8301
CAS No.:15401-60-2
- Carmustine
Catalog No.:BCC5244
CAS No.:154-93-8
- Bz-Arg-OH
Catalog No.:BCC2856
CAS No.:154-92-7
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
Investigating antimalarial drug interactions of emetine dihydrochloride hydrate using CalcuSyn-based interactivity calculations.[Pubmed:28257497]
PLoS One. 2017 Mar 3;12(3):e0173303.
The widespread introduction of artemisinin-based combination therapy has contributed to recent reductions in malaria mortality. Combination therapies have a range of advantages, including synergism, toxicity reduction, and delaying the onset of resistance acquisition. Unfortunately, antimalarial combination therapy is limited by the depleting repertoire of effective drugs with distinct target pathways. To fast-track antimalarial drug discovery, we have previously employed drug-repositioning to identify the anti-amoebic drug, emetine dihydrochloride hydrate, as a potential candidate for repositioned use against malaria. Despite its 1000-fold increase in in vitro antimalarial potency (ED50 47 nM) compared with its anti-amoebic potency (ED50 26-32 uM), practical use of the compound has been limited by dose-dependent toxicity (emesis and cardiotoxicity). Identification of a synergistic partner drug would present an opportunity for dose-reduction, thus increasing the therapeutic window. The lack of reliable and standardised methodology to enable the in vitro definition of synergistic potential for antimalarials is a major drawback. Here we use isobologram and combination-index data generated by CalcuSyn software analyses (Biosoft v2.1) to define drug interactivity in an objective, automated manner. The method, based on the median effect principle proposed by Chou and Talalay, was initially validated for antimalarial application using the known synergistic combination (atovaquone-proguanil). The combination was used to further understand the relationship between SYBR Green viability and cytocidal versus cytostatic effects of drugs at higher levels of inhibition. We report here the use of the optimised Chou Talalay method to define synergistic antimalarial drug interactivity between emetine dihydrochloride hydrate and atovaquone. The novel findings present a potential route to harness the nanomolar antimalarial efficacy of this affordable natural product.
Original research paper. Characterization and taste masking evaluation of microparticles with cetirizine dihydrochloride and methacrylate-based copolymer obtained by spray drying.[Pubmed:28231047]
Acta Pharm. 2017 Mar 1;67(1):113-124.
Taste of a pharmaceutical formulation is an important parameter for the effectiveness of pharmacotherapy. Cetirizine dihydrochloride (CET) is a second-generation antihistamine that is commonly administered in allergy treatment. CET is characterized by extremely bitter taste and it is a great challenge to successfully mask its taste; therefore the goal of this work was to formulate and characterize the microparticles obtained by the spray drying method with CET and poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate 1:2:1 copolymer (Eudragit E PO) as a barrier coating. Assessment of taste masking by the electronic tongue has revealed that designed formulations created an effective taste masking barrier. Taste masking effect was also confirmed by the in vivo model and the in vitro release profile of CET. Obtained data have shown that microparticles with a drug/polymer ratio (0.5:1) are promising CET carriers with efficient taste masking potential and might be further used in designing orodispersible dosage forms with CET.
Pretreatment cognitive and neural differences between sapropterin dihydrochloride responders and non-responders with phenylketonuria.[Pubmed:28271047]
Mol Genet Metab Rep. 2017 Feb 23;12:8-13.
Sapropterin dihydrochloride (BH4) reduces phenylalanine (Phe) levels and improves white matter integrity in a subset of individuals with phenylketonuria (PKU) known as "responders." Although prior research has identified biochemical and genotypic differences between BH4 responders and non-responders, cognitive and neural differences remain largely unexplored. To this end, we compared intelligence and white matter integrity prior to treatment with BH4 in 13 subsequent BH4 responders with PKU, 16 subsequent BH4 non-responders with PKU, and 12 healthy controls. Results indicated poorer intelligence and white matter integrity in non-responders compared to responders prior to treatment. In addition, poorer white matter integrity was associated with greater variability in Phe across the lifetime in non-responders but not in responders. These results underscore the importance of considering PKU as a multi-faceted, multi-dimensional disorder and point to the need for additional research to delineate characteristics that predict response to treatment with BH4.
Proposed phase 2/ step 2 in-vitro test on basis of EN 14561 for standardised testing of the wound antiseptics PVP-iodine, chlorhexidine digluconate, polihexanide and octenidine dihydrochloride.[Pubmed:28193164]
BMC Infect Dis. 2017 Feb 13;17(1):143.
BACKGROUND: Currently, there is no agreed standard for exploring the antimicrobial activity of wound antiseptics in a phase 2/ step 2 test protocol. In the present study, a standardised in-vitro test is proposed, which allows to test potential antiseptics in a more realistically simulation of conditions found in wounds as in a suspension test. Furthermore, factors potentially influencing test results such as type of materials used as test carrier or various compositions of organic soil challenge were investigated in detail. METHODS: This proposed phase 2/ step 2 test method was modified on basis of the EN 14561 by drying the microbial test suspension on a metal carrier for 1 h, overlaying the test wound antiseptic, washing-off, neutralization, and dispersion at serial dilutions at the end of the required exposure time yielded reproducible, consistent test results. RESULTS: The difference between the rapid onset of the antiseptic effect of PVP-I and the delayed onset especially of polihexanide was apparent. Among surface-active antimicrobial compounds, octenidine was more effective than chlorhexidine digluconate and polihexanide, with some differences depending on the test organisms. However, octenidine and PVP-I were approximately equivalent in efficiency and microbial spectrum, while polihexanide required longer exposure times or higher concentrations for a comparable antimicrobial efficacy. CONCLUSION: Overall, this method allowed testing and comparing differ liquid and gel based antimicrobial compounds in a standardised setting.
Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice.[Pubmed:9600576]
Psychopharmacology (Berl). 1998 Apr;136(4):320-7.
Abnormal sensory inhibition is a measurable indicator of a sensory processing deficit which is observed in schizophrenia, and other disorders, and which may be heritable. This deficit has also been observed in certain inbred mouse strains where the intensity of the deficit has been correlated with reduction in the number of hippocampal alpha-bungarotoxin-sensitive nicotinic receptors. Nicotine and certain nicotinic agonists produce brief periods of normal sensory inhibition in these mice. Similarly, nicotine also transiently normalizes sensory inhibition in schizophrenics. The present study assessed the effects of a novel nicotinic partial agonist (GTS-21), selective for the alpha-bungarotoxin site, on sensory inhibition in DBA mice, a strain with no sensory inhibition under routine experimental conditions. GTS-21 produced a dose-dependent normalization of sensory inhibition which was blocked by alpha-bungarotoxin but not mecamylamine. In contrast to other nicotinic agonists, normalization of sensory inhibition by GTS-21 and two related anabaseine compounds, DMAB-anabaseine and DMAC-anabaseine, was observed when administered a second time to the animal, after a 40-min delay. Our results indicated that the anabaseine compounds increase sensory inhibition through alpha7 nicotinic receptors, and that their ability to act repeatedly on these receptors may be less affected by desensitization.
Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors.[Pubmed:9399967]
J Pharmacol Exp Ther. 1997 Dec;283(3):979-92.
We assessed the pharmacological activity of anabaseine, a toxin found in certain animal venoms, relative to nicotine and anabasine on a variety of vertebrate nicotinic receptors, using cultured cells, the Xenopus oocyte expression system, contractility assays with skeletal and smooth muscle strips containing nicotinic receptors and in vivo rat prostration assay involving direct injection into the lateral ventricle of the brain. Anabaseine stimulated every subtype of nicotinic receptor that was tested. It was the most potent frog skeletal muscle nicotinic receptor agonist. At higher concentrations it also blocked the BC3H1 (adult mouse) muscle type receptor ion channel. The affinities of the three nicotinoid compounds for rat brain membrane alpha-bungarotoxin binding sites and their potencies for stimulating Xenopus oocyte homomeric alpha7 receptors, expressed in terms of their active monocation concentrations, displayed the same rank order, anabaseine>anabasine> nicotine. Although the maximum currents generated by anabaseine and anabasine at alpha7 receptors were equivalent to that of acetylcholine, the maximum response to nicotine was only about 65% of the acetylcholine response. At alpha4-beta2 receptors the affinities and apparent efficacies of anabaseine and anabasine were much less than that of nicotine. Anabaseine, nicotine and anabasine were nearly equipotent on sympathetic (PC12) receptors, although parasympathetic (myenteric plexus) receptors were much more sensitive to anabaseine and nicotine but less sensitive to anabasine. These differences suggest that there may be different subunit combinations in these two autonomic nicotinic receptors. The preferential interactions of anabaseine, anabasine and nicotine with different receptor subtypes provides molecular clues that should be helpful in the design of selective nicotinic agonists.
Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21.[Pubmed:7796104]
Brain Res. 1995 Mar 20;674(2):252-9.
The ability of two synthetic nicotine receptor ligands, TGS-21 and DMAB, to chronically enhance the cognitive function of aged rats was evaluated in three diverse tasks and compared to the cognition-enhancing effects of nicotine administration. 15 min prior to daily behavioral testing, aged 22-24 month old rats received an i.p. injection of nicotine (.02 mg/kg), GTS-21 (1 mg/kg), DMAB (2mg/kg), or saline vehicle and were tested in either one-way active avoidance pole jumping, Lashley III maze, or a 17-arm radial maze. GTS-21 pretreatment was as effective as nicotine for enhancing the acquisition of aged rats in both one-way active avoidance and Lashley III maze training. In 17-arm radial maze testing, GTS-21 improved both general learning and reference (long-term) memory to the same extent as nicotine. Although DMAB pretreatment enhanced reference memory in 17-arm radial maze testing to the same as nicotine, it did not affect general learning in this complex task and did not exert any cognition-enhancing effects in Lashley III maze training. These results indicate that GTS-21 has cognition-enhancing abilities in aged rats that are comparable to those of nicotine in a variety of behavioral tasks. Since GTS-21 acts preferentially on brain nicotinic receptors and is less toxic than nicotine, thses results further indicate that GTS-21 may have substantive therapeutic value in the treatment of age-associated memory impairment (AAMI) and/or Alzheimer's disease.


