CarmustineCAS# 154-93-8 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154-93-8 | SDF | Download SDF |
| PubChem ID | 2578 | Appearance | Powder |
| Formula | C5H9Cl2N3O2 | M.Wt | 214.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 35 mg/mL (163.51 mM) *"≥" means soluble, but saturation unknown. | ||
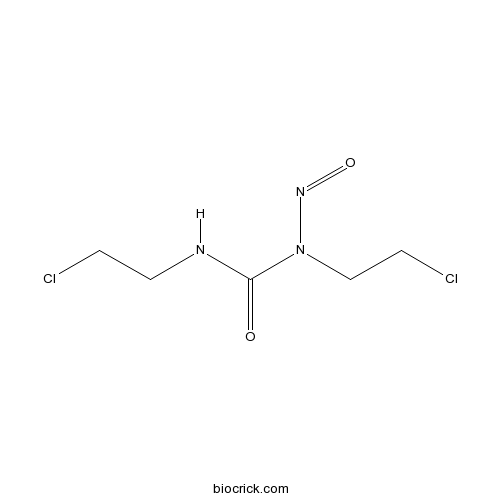
| Chemical Name | 1,3-bis(2-chloroethyl)-1-nitrosourea | ||
| SMILES | C(CCl)NC(=O)N(CCCl)N=O | ||
| Standard InChIKey | DLGOEMSEDOSKAD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H9Cl2N3O2/c6-1-3-8-5(11)10(9-12)4-2-7/h1-4H2,(H,8,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Carmustine Dilution Calculator

Carmustine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6718 mL | 23.359 mL | 46.7181 mL | 93.4361 mL | 116.7951 mL |
| 5 mM | 0.9344 mL | 4.6718 mL | 9.3436 mL | 18.6872 mL | 23.359 mL |
| 10 mM | 0.4672 mL | 2.3359 mL | 4.6718 mL | 9.3436 mL | 11.6795 mL |
| 50 mM | 0.0934 mL | 0.4672 mL | 0.9344 mL | 1.8687 mL | 2.3359 mL |
| 100 mM | 0.0467 mL | 0.2336 mL | 0.4672 mL | 0.9344 mL | 1.168 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Carmustine is an antitumor chemotherapeutic agent, which works by akylating DNA and RNA.
In Vitro:Carmustine is an antitumor chemotherapeutic agent. Carmustine (8, 80, and 800 µM) decreases N-acetyltransferase (NAT) activities for 2-aminofluorene (AF) and p-aminobenzoic acid (PABA) in rat glial tumor cytosol and intact cells. In rat glial tumor cells, the DNA-AF adduct increases, and carmustine decreases the formation of DNA-AF adduct[1].
In Vivo:Carmustine (BCNU; 25 mg/kg, i.p.) causes higher levels of the rhe ratio of liver weight to body weight and plasma conjugated bilirubin, and lower biliary flow, oxidised glutation levels (GSSG) and reduced glutation (GSH)/GSSG values compared with control rats[2].
References:
[1]. Hung CF. Effects of carmustine and lomustine on arylamine N-acetyltransferase activity and 2-aminofluorene-DNA adducts in rat glial tumor cells. Neurochem Res. 2000 Jun;25(6):845-51.
[2]. Demir A, et al. The effect of trimetazidine on intrahepatic cholestasis caused by carmustine in rats. Hepatol Res. 2001 May 1;20(1):133-143.
- Bz-Arg-OH
Catalog No.:BCC2856
CAS No.:154-92-7
- Tripelennamine HCl
Catalog No.:BCC4523
CAS No.:154-69-8
- Primulaverin
Catalog No.:BCC8235
CAS No.:154-61-0
- Primverin
Catalog No.:BCC8238
CAS No.:154-60-9
- 1,5-Anhydro-D-glucitol
Catalog No.:BCN2234
CAS No.:154-58-5
- Thioguanine
Catalog No.:BCC2220
CAS No.:154-42-7
- Catechin
Catalog No.:BCN1688
CAS No.:154-23-4
- Lincomycin
Catalog No.:BCC9010
CAS No.:154-21-2
- 2-Deoxy-D-glucose
Catalog No.:BCC4048
CAS No.:154-17-6
- ANQ 11125
Catalog No.:BCC6359
CAS No.:153966-48-4
- p-Hydroxyphenethyl vanillate
Catalog No.:BCN7555
CAS No.:1539303-03-1
- NBOH-2C-CN hydrochloride
Catalog No.:BCC8025
CAS No.:1539266-32-4
- Ipecoside
Catalog No.:BCN8301
CAS No.:15401-60-2
- Berberrubine
Catalog No.:BCN2651
CAS No.:15401-69-1
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
Polifeprosan 20, 3.85% carmustine slow release wafer in malignant glioma: patient selection and perspectives on a low-burden therapy.[Pubmed:27920506]
Patient Prefer Adherence. 2016 Nov 24;10:2397-2406.
Polifeprosan 20 with Carmustine (GLIADEL((R))) polymer implant wafer is a biodegradable compound containing 3.85% Carmustine (BCNU, bischloroethylnitrosourea) implanted in the brain at the time of planned tumor surgery, which then slowly degrades to release the BCNU chemotherapy directly into the brain thereby bypassing the blood-brain barrier. Carmustine implant wafers were demonstrated to improve survival in randomized placebo-controlled trials in patients undergoing a near total resection of newly diagnosed or recurrent malignant glioma. Based on these trials and other supporting data, Carmustine wafer therapy was approved for use for newly diagnosed and recurrent malignant glioma in the United States and the European Union. Adverse events are uncommon, and as this therapy is placed at the time of surgery, it does not add to patient treatment burden. Nevertheless, this therapy appears to be underutilized. This article reviews the evidence for a favorable therapeutic ratio for the patient and the potential barriers. Consideration of these issues is important for optimal use of this therapeutic approach and may be important as this technology and other local therapies are further developed in the future.
Mathematical Modelling of Convection Enhanced Delivery of Carmustine and Paclitaxel for Brain Tumour Therapy.[Pubmed:28155074]
Pharm Res. 2017 Apr;34(4):860-873.
PURPOSE: Convection enhanced delivery (CED) is a promising method of anticancer treatment to bypass the blood-brain barrier. This paper is aimed to study drug transport under different CED operating conditions. METHODS: The convection enhanced delivery of chemotherapeutics to an intact and a remnant brain tumour after resection is investigated by means of mathematical modelling of the key physical and physiological processes of drug transport. Realistic models of brain tumour and its holding tissue are reconstructed from magnetic resonance images. Mathematical modelling is performed for the delivery of Carmustine and paclitaxel with different infusion rates, solution concentrations and locations of infusion site. RESULTS: Modelling predications show that drug penetration can be improved by raising the infusion rate and the infusion solution concentration. The delivery of Carmustine with CED is highly localised. High drug concentration only can be achieved around the infusion site. The transport of paclitaxel is more sensitive to CED-enhanced interstitial fluid as compared to Carmustine, with deeper penetration into tumour interior. Infusing paclitaxel in the upstream of interstitial fluid flow leads to high spatial averaged concentration and relatively uniform distribution. CONCLUSION: Results obtained in this study can be used to guide the design and optimisation of CED treatment regimens.
Evaluation of O6-Benzylguanine-Potentiated Topical Carmustine for Mycosis Fungoides: A Phase 1-2 Clinical Trial.[Pubmed:28199478]
JAMA Dermatol. 2017 May 1;153(5):413-420.
Importance: In a phase 1 trial, single-dose O6-benzylguanine with topical Carmustine for patients with early stage (stage IA through stage IIA) cutaneous T-cell lymphoma, mycosis fungoides (MF) type, resulted in clinical responses proportional to inhibition of O6-alkylguanine-DNA alkyltransferase activity, but a maximum tolerated dose (MTD) was not reached. Objective: To determine whether dose escalation of Carmustine in combination with dual-dose O6-benzylguanine to prolong alkyltransferase inhibition could reach an MTD. Design, Setting, and Participants: A single-arm, phase 1-2 clinical trial conducted at a university teaching hospital enrolled 17 adults with stage IA through stage IIA cutaneous T-cell lymphoma, MF type, to evaluate treatment using topical Carmustine plus 2 subsequent daily doses of intravenous O6-benzylguanine, administered every 2 weeks for up to 24 weeks (12 cycles). All patients who received treatment were included in an intent-to-treat analysis of the response rate. The study was conducted from February 17, 2010, to April 8, 2014. Data analysis was performed from May 1, 2014, to December 1, 2015. Interventions: Topical Carmustine and intravenous O6-benzylguanine. Main Outcomes and Measures: Clinical disease response was assessed by the Severity-Weighted Assessment Tool (score range, 0-400; higher score indicates worse disease). Safety data were acquired by review of adverse events at study visits. Results: Of the 17 patients enrolled, 12 (71%) were men; mean (SD) age was 45.2 (14.6) years. There were 7 complete responses and 8 partial responses to combination Carmustine and O6-benzylguanine treatment. The overall clinical response rate was 88%, with a mean (SD) duration of complete response of 14.43 (6.6) months. The MTD was 20 mg of Carmustine applied once in combination with 2 daily doses of 120 mg/m2 of O6-benzylguanine. Most adverse events (112 [67%]) were grade I. Of 15 patients with dermatitis, 5 individuals (33%) demonstrated grade II dermatitis that was unresponsive to topical corticosteroid therapy. The dermatitis was characterized by high levels of macrophage activation, and clearance was associated with vitamin D3 administration. Conclusions and Relevance: Compared with single-dose O6-benzylguanine and Carmustine, dual-dose O6-benzylguanine resulted in higher overall response rates and reduced total Carmustine doses but was associated with more cutaneous adverse events. The MTD for dual-dose O6-benzylguanine plus Carmustine was also ascertained. Trial Registration: clinicaltrials.gov Identifier: NCT00961220.
Carmustine replacement in intensive chemotherapy preceding reinjection of autologous HSCs in Hodgkin and non-Hodgkin lymphoma: a review.[Pubmed:28112752]
Bone Marrow Transplant. 2017 Jul;52(7):941-949.
High-dose chemotherapy preceding autologous hematopoietic stem cell transplantation (auto-HSCT) is one treatment option for patients with Hodgkin (HL) or non-Hodgkin lymphoma (NHL). The most frequently used intensive chemotherapy is a combination of Carmustine (BCNU), etoposide, cytarabine and melphalan (BEAM). However, BCNU is consistently in short supply, and there has been a recent dramatic increase in its cost, necessitating the utilization of conditioning alternatives. The busulfan-based conditioning regimen known as the busulfan-cyclophosphamide-etoposide (BuCyE) combination is the second most-studied conditioning regimen worldwide after BEAM, and it exhibits a benefit/risk ratio that is comparable to that of BEAM. In addition to these two combinations, the present manuscript also summarizes data reported for other conditioning combinations. Owing to the lack of prospective and comparative studies, a comparison of the toxicities and medicoeconomical profiles of these treatments is warranted to identify effective replacements for BCNU-based conditioning.


