2-Deoxy-D-glucoseGlycolysis inhibitor CAS# 154-17-6 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154-17-6 | SDF | Download SDF |
| PubChem ID | 40 | Appearance | Powder |
| Formula | C6H12O5 | M.Wt | 164.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 2-Deoxy-D-arabino-hexose; D-Arabino-2-deoxyhexose | ||
| Solubility | DMSO : ≥ 51 mg/mL (310.67 mM) H2O : ≥ 24 mg/mL (146.20 mM) *"≥" means soluble, but saturation unknown. | ||
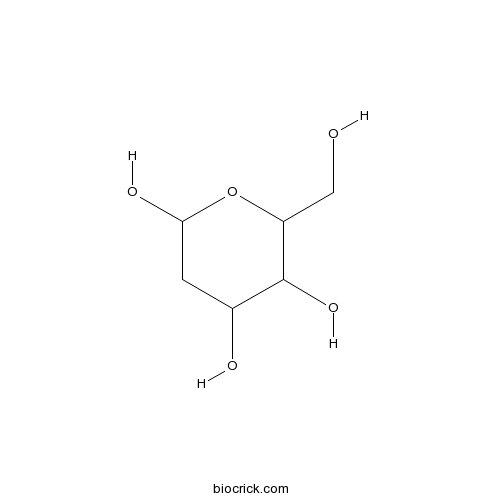
| Chemical Name | 6-(hydroxymethyl)oxane-2,4,5-triol | ||
| SMILES | C1C(C(C(OC1O)CO)O)O | ||
| Standard InChIKey | PMMURAAUARKVCB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H12O5/c7-2-4-6(10)3(8)1-5(9)11-4/h3-10H,1-2H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-metabolizable glucose analog. Inhibits phosphorylation of glucose by hexokinase; causes depletion of cellular ATP. Also inhibits phosphoglucose isomerase (PGI) competitively. Causes cell cycle inhibition and cell death in in vitro models of hypoxia; blocks tumor cell growth in animal models. Also shown to induce the unfolded protein response (UPR). |

2-Deoxy-D-glucose Dilution Calculator

2-Deoxy-D-glucose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0916 mL | 30.4581 mL | 60.9162 mL | 121.8324 mL | 152.2904 mL |
| 5 mM | 1.2183 mL | 6.0916 mL | 12.1832 mL | 24.3665 mL | 30.4581 mL |
| 10 mM | 0.6092 mL | 3.0458 mL | 6.0916 mL | 12.1832 mL | 15.229 mL |
| 50 mM | 0.1218 mL | 0.6092 mL | 1.2183 mL | 2.4366 mL | 3.0458 mL |
| 100 mM | 0.0609 mL | 0.3046 mL | 0.6092 mL | 1.2183 mL | 1.5229 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
2-Deoxy-D-glucose (2DG), glucose analogue, is a competitive glycolytic inhibitor [1] [2] [3]. Cytotoxicity assays displayed IC50 values of 2DG in KIT-positive GIST cell lines between 0.5μM (GIST882 cell line) and 2.5μM (GIST430 cell line) [4].
Glycolysis is an ATP-producing subsystem [5].
If the physiologically relevant ratio of 2DG/glucose is 0.8, the glucose metabolism in the FaDu cells that are growing in DMEM containing 25 mmol/L glucose will be inhibited. Treatment with 2DG caused a 30% to 40% decrease in total glutathione content and 32% cell killing relative to untreated control cells [2]. 2-DG inhibited PEDV propagation in a concentration-dependent manner and induced endoplasmic reticulum stress in Vero cells. The 2-DG treatment did not significantly affect virus entry. But further research showed that 2-DG decreased viral protein translation, implying that 2-DG might affect virus replication at the early stage of virus infection. Additionally, the 2-DG treatment increased the expression level of CD13 [6].
2-Deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo in retarding tumor growth and prolonging survival [3]. In Sparus aurata L., 2-DG increased blood glucose levels in a statistically significant way, caused a non-statistically significant decrease in the respiratory burst activity of head kidney leucocytes. Peroxidase activity measured in serum and head kidney leukocyte showed no statistically significant difference with respect to the control values [7].
References:
[1]. John Laszlo, William R. Harlan, Robert F. Klein, et al. The Effect of 2-Deoxy-D-Glucose Infusions on Lipid and Carbohydrate Metabolism in Man. J Clin Invest., 1961, 40(1):171-176.
[2]. Andrean L. Simons, Iman M. Ahmad, David M. Mattson, et al. 2-Deoxy-D-Glucose Combined with Cisplatin Enhances Cytotoxicity via Metabolic Oxidative Stress in Human Head and NeckCancer Cells. Cancer Research, 2007, 67(7): 3364-3371.
[3]. Gregory Maschek, Niramol Savaraj, Waldemar Priebe, et al. 2-Deoxy-D-glucose Increases the Efficacy of Adriamycin and Paclitaxel in Human Osteosarcoma and Non-Small Cell Lung Cancers In Vivo. Cancer Research, 2004, 64:31-34.
[4]. Thomas Mühlenberg, Susanne Grunewald, Jürgen Treckmann, et al. Inhibition of KIT-Glycosylation by 2-Deoxyglucose Abrogates KIT-Signaling and Combination with ABT-263 Synergistically Induces Apoptosis in Gastrointestinal Stromal Tumor. PLOS ONE, 2015, 10(3):e0120531.
[5]. R. J. Connett, C. R. Honig, T. E. Gayeski, et al. Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. Journal of Applied Physiology, 1990, 68(3): 833-842.
[6]. Yue Wang, Jia-rong Li, Ming-xia Sun, et al. Triggering unfolded protein response by 2-Deoxy-D-glucose inhibits porcine epidemic diarrhea virus propagation. Antiviral Research, 2014, 106: 33–41.
[7]. F.A. Guardiola, R. Cerezuela, J. Meseguer, et al. Effects of 2-deoxy-D-glucose on the immune system of seabream (Sparus aurata L.). Fish & Shellfish Immunology, 2011, 30: 592-599.
- ANQ 11125
Catalog No.:BCC6359
CAS No.:153966-48-4
- p-Hydroxyphenethyl vanillate
Catalog No.:BCN7555
CAS No.:1539303-03-1
- NBOH-2C-CN hydrochloride
Catalog No.:BCC8025
CAS No.:1539266-32-4
- Boc-Gln-ONp
Catalog No.:BCC3383
CAS No.:15387-45-8
- BLU9931
Catalog No.:BCC3979
CAS No.:1538604-68-0
- Fmoc-Trp-ol
Catalog No.:BCC2573
CAS No.:153815-60-2
- Dioxopromethazine hydrochloride
Catalog No.:BCC8946
CAS No.:15374-15-9
- Eriodictyol-8-glucoside
Catalog No.:BCN8029
CAS No.:153733-96-1
- 9,10-Bis(3,5-dihydroxyphenyl)anthracene
Catalog No.:BCC8793
CAS No.:153715-08-3
- H-Ile-OEt.HCl
Catalog No.:BCC2961
CAS No.:15366-32-3
- Dofequidar fumarate
Catalog No.:BCC4177
CAS No.:153653-30-6
- GNE-9605
Catalog No.:BCC5458
CAS No.:1536200-31-3
- Lincomycin
Catalog No.:BCC9010
CAS No.:154-21-2
- Catechin
Catalog No.:BCN1688
CAS No.:154-23-4
- Thioguanine
Catalog No.:BCC2220
CAS No.:154-42-7
- 1,5-Anhydro-D-glucitol
Catalog No.:BCN2234
CAS No.:154-58-5
- Primverin
Catalog No.:BCC8238
CAS No.:154-60-9
- Primulaverin
Catalog No.:BCC8235
CAS No.:154-61-0
- Tripelennamine HCl
Catalog No.:BCC4523
CAS No.:154-69-8
- Bz-Arg-OH
Catalog No.:BCC2856
CAS No.:154-92-7
- Carmustine
Catalog No.:BCC5244
CAS No.:154-93-8
- Ipecoside
Catalog No.:BCN8301
CAS No.:15401-60-2
- Berberrubine
Catalog No.:BCN2651
CAS No.:15401-69-1
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
Potential excitatory role of nitric oxide on 2-deoxy-d-glucose-induced gastric motility in rats.[Pubmed:28294385]
Clin Exp Pharmacol Physiol. 2017 Jun;44(6):693-699.
Previous studies have shown that 2-Deoxy-D-glucose (2-DG) increases gastric motility via the vagus nerve, but the underlying mechanism remains elusive. Since nitric oxide (NO) is involved in gastric motility, a possible interplay between 2-DG and NO can be suggested. In the present study, Wistar rats (250-350 g) of both sexes were intravenously injected with 2-DG (200 mg/kg), and the effects of the intravenous injection of the nitric oxide synthase (NOS) inhibitors; nitro-l-arginine methyl ester (l-NAME, 10 mg/kg) and N(omega) -nitro-l-arginine (l-NNA, 10 mg/kg) were investigated. Animals were anaesthetized and cannulated for intravenous drug injections while the left vagal nerve was electrically stimulated (0.1-10 Hz, 0.5 ms duration, 12 V, for 60 seconds), and intragastric pressure and gastric motility changes were monitored using a latex gastric balloon. 2-DG increased the mean intragastric pressure (baseline, 5.0+/-0.4 cmH2 O; after 2-DG, 14.4+/-1.5 cmH2 O; P=.0156) and significantly increased the gastric motility index, while NOS inhibitors significantly attenuated both parameters. However, pretreatment with NOS inhibitors significantly augmented the gastric responses to peripheral electrical vagal stimulation. These results suggest that NO plays an excitatory role in gastric responsiveness to 2-DG and that this function may be effected in the central nervous system.
All That Glitters Is Not Gold" - A Case of an Occult Foreign Body in the Lung with Elevated 2-[18F]-Fluoro-2-deoxy-D-glucose (FDG) Uptake Mimicking Bronchogenic Carcinoma.[Pubmed:28265526]
Cureus. 2017 Jan 23;9(1):e990.
Combined positron emission tomography/computed tomography (PET/CT) using the glucose analogue 2-[18F]-fluoro-2-Deoxy-D-glucose (FDG) has become the standard of care in oncological patients. However, due to the non-specific nature of FDG uptake, there are many physiological variants and benign pathological entities that also demonstrate augmented glucose metabolism, such as inflammatory and infective processes. Undiagnosed and retained foreign bodies (occult foreign bodies) in the lung can induce inflammatory reaction consisting of polymorphonuclear neutrophils, macrophages, and granulation tissue resulting in intense FDG uptake because of high metabolic activity and cell turnover. Here, we present a case of an occult foreign body imitating a tumor on PET/CT.
Co-treatment of breast cancer cells with pharmacologic doses of 2-deoxy-D-glucose and metformin: Starving tumors.[Pubmed:28350075]
Oncol Rep. 2017 Apr;37(4):2418-2424.
A characteristic of tumor cells is the increased aerobic glycolysis for energy production. Thus, inhibition of glycolysis represents a selective therapeutic option. It has been shown that glycolysis inhibitor 2-Deoxy-D-glucose (2DG) induces apoptotic cell death in different tumor entities. In addition, the antitumor activity of the anti-diabetic drug metformin has been demonstrated. In the present study, we aimed to ascertain whether the combination of pharmacologic doses of 2DG with metformin increases the antitumor efficacy. Cell viability of MDA-MB-231 and HCC1806 triple-negative breast cancer (TNBC) cells treated without or with 2DG or with metformin alone or with the combination of both agents was measured using Alamar Blue assay. Induction of apoptosis was quantified by measurement of the loss of mitochondrial membrane potential and cleavage of PARP. Treatment of breast cancer cells with glycolysis inhibitor 2DG or with the anti-diabetic drug metformin resulted in a significant decrease in cell viability and an increase in apoptosis. Treatment with 2DG in combination with metformin resulted in significantly reduced viability compared with the single agent treatments. The observed reduction in viability was due to induction of apoptosis. In addition, in regards to apoptosis induction a stronger effect in the case of co-treatment compared with single agent treatments was observed. The glycolytic phenotype of human breast cancer cells can be targeted for therapeutic intervention. Co-treatment with doses of the glycolysis inhibitor 2DG and anti-diabetic drug metformin is tolerable in humans and may be a suitable therapy for human breast cancers.
Short day length enhances physiological resilience of the immune system against 2-deoxy-d-glucose-induced metabolic stress in a tropical seasonal breeder Funambulus pennanti.[Pubmed:28131595]
Horm Behav. 2017 Mar;89:157-166.
Studies demonstrate the importance of metabolic resources in the regulation of reproduction and immune functions in seasonal breeders. In this regard, the restricted energy availability can be considered as an environmental variable that may act as a seasonal stressor and can lead to compromised immune functions. The present study explored the effect of photoperiodic variation in the regulation of immune function under metabolic stress condition. The T-cell-dependent immune response in a tropical seasonal breeder Funambulus pennanti was studied following the inhibition of cellular glucose utilization with 2-Deoxy-D-glucose (2-DG). 2-DG treatment resulted in the suppression of general (e.g., proliferative response of lymphocytes) and antigen-specific [anti-keyhole limpet hemocyanin IgG titer and delayed-type hypersensitivity response] T-cell responses with an activation of the hypothalamic-pituitary-adrenal axis, which was evident from the increased levels of plasma corticosterone. 2-DG administration increased the production of inflammatory cytokines [interleukin (IL)-1beta and tumor necrosis factor (TNF)-alpha] and decreased the autocrine T-cell growth factor IL-2. The immunocompromising effect of 2-DG administration was retarded in animals exposed to short photoperiods compared with the control and long photoperiod-exposed groups. This finding suggested that short photoperiodic conditions enhanced the resilience of the immune system, possibly by diverting metabolic resources from the reproductive organs toward the immune system. In addition, melatonin may have facilitated the energy "trade-off" between reproductive and immune mechanisms, thereby providing an advantage to the seasonal breeders for their survival during stressful environmental conditions.
2-Deoxyglucose: an anticancer and antiviral therapeutic, but not any more a low glucose mimetic.[Pubmed:16111712]
Life Sci. 2006 Feb 16;78(12):1392-9.
2-Deoxyglucose (2-DG), a non-metabolizable glucose analogue, blocks glycolysis and inhibits protein glycosylation. It has been tested in multiple studies for possible application as an anticancer or antiviral therapeutic. The inhibitory effect of 2-DG on ATP generation made it a good candidate molecule as a calorie restriction mimetic as well. Furthermore, 2-DG has been utilized in numerous studies to simulate a condition of glucose starvation. Because 2-DG disrupts glucose metabolism, protein glycosylation, and ER quality control at the same time, a cellular or pathologic outcome could be easily misinterpreted without clear understanding of 2-DG's effect on each of these aspects. However, the effect of 2-DG on protein glycosylation has rarely been investigated. A recent study suggested that 2-DG causes hyperGlcNAcylation of proteins, while low glucose supply causes hypoGlcNAcylation. In certain aspects of cellular physiology, this difference could be disregarded, but in others, this may possibly cause totally different outcomes.
Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions.[Pubmed:14605866]
Cancer Chemother Pharmacol. 2004 Feb;53(2):116-22.
PURPOSE: In order to investigate the hypothesis that cells found in hypoxic areas of solid tumors are more sensitive to glycolytic inhibitors than cells growing aerobically, we have previously characterized three distinct in vitro models (A, B and C) that simulate this condition. In all of the models it was shown that cells growing under hypoxic conditions are hypersensitive to the glycolytic inhibitor 2-deoxy- d-glucose (2-DG). However, in those studies cytostatic and cytotoxic effects were not distinguished from one another. Since successful treatment of cancer includes not only slowing down but also actually killing tumor cells, studies were undertaken to assess the effects of 2-DG on cell cycle progression and cell death. METHODS AND RESULTS: Using flow cytometry and cell viability assays, it was found that 2-DG caused significantly greater cell cycle inhibition and cell death in all three hypoxic models as compared to aerobically growing control cells. In model A (a chemically induced model of hypoxia in which rhodamine-123 is used to block oxidative phosphorylation), 1200 microg/ml of 2-DG was shown to induce more cell cycle arrest in late S/G(2) and more cell death than in the aerobic cell counterpart treated with 3600 microg/ml 2-DG. In rho(0) cells which are genetically constructed to be unable to perform oxidative phosphorylation (model B), an even greater window of selectivity (more than tenfold) between hypoxic and aerobic cells was found when considering 2-DG's effects on cell cycle arrest and cell death. In the environmental model (model C), where cells were grown under reduced amounts of external oxygen (0.1%), hypersensitivity to the effects of 2-DG with respect to cell cycle arrest and cell death were also observed. CONCLUSIONS: Overall, these results indicate that cells growing under anaerobic conditions respond with greater sensitivity to the effects of 2-DG on cell cycle inhibition and cell death than those growing under aerobic conditions. This supports our contention that glycolytic inhibitors added to standard chemotherapeutic protocols should increase treatment efficacy by selectively killing the slow-growing cells, which are found in the hypoxic portions of solid tumors, while sparing most of the normal cells that are also slow-growing but are living under aerobic conditions.


