CatechinCAS# 154-23-4 |
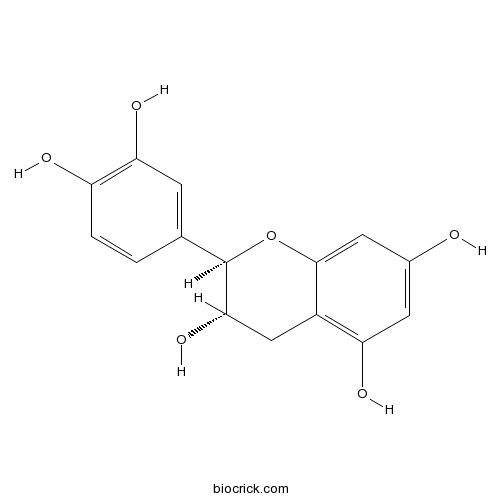
- Epicatechin
Catalog No.:BCN5597
CAS No.:490-46-0
- DL-Catechin
Catalog No.:BCN6325
CAS No.:7295-85-4
- (-)-Catechin
Catalog No.:BCN0308
CAS No.:18829-70-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154-23-4 | SDF | Download SDF |
| PubChem ID | 9064 | Appearance | Cream-coloured powder |
| Formula | C15H14O6 | M.Wt | 290.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | (+)-Catechin; Cianidanol; D-Catechin; Cyanidanol; Catechuic acid;7295-85-4 | ||
| Solubility | DMSO : ≥ 100 mg/mL (344.51 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | ||
| SMILES | C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C=C3)O)O)O | ||
| Standard InChIKey | PFTAWBLQPZVEMU-DZGCQCFKSA-N | ||
| Standard InChI | InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Catechin, a cyclooxygenase-1 (COX-1) inhibitor with an IC50 of 1.4 μM, which has antiangiogenic, antitumor, antioxidant, UV-protective, anti-aging, phytotoxic, antimicrobial, and antiviral effects. Catechin shows its potential as biobased active packaging for fatty food, and exerts cardioprotection through treating many kinds of angiocardiopathy. |
| Targets | IL Receptor | JAK | STAT | MAPK | PI3K | Akt | COX-1 | Antifection |
| In vitro | Effect of emulsification on the skin permeation and UV protection of catechin.[Pubmed: 23639253]Pharm Dev Technol. 2014 Jun;19(4):395-400.An anti-aging effect may be obtained by skin application of tea Catechins (Camellia sinensis) since they have high ultraviolet (UV)-protection activity.
Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications.[Pubmed: 21827739 ]Biochem Pharmacol. 2011 Dec 15;82(12):1807-21.An expanding body of preclinical evidence suggests EGCG, the major Catechin found in green tea (Camellia sinensis), has the potential to impact a variety of human diseases.
|
| In vivo | Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study.[Pubmed: 11470725]Am J Clin Nutr. 2001 Aug;74(2):227-32.Epidemiologic studies suggest that tea consumption may reduce the risk of cardiovascular diseases, but results are inconsistent. Catechins, which belong to the flavonoid family, are the main components of tea and may be responsible for the alleged protective effect. Taking Catechin sources other than tea into account might clarify the reported associations.
|
| Cell Research | Antiviral effect of catechins in green tea on influenza virus.[Pubmed: 16137775 ]Antiviral Res. 2005 Nov;68(2):66-74.Polyphenolic compound Catechins ((-)-epigalloCatechin gallate (EGCG), (-)-epiCatechin gallate (ECG) and (-)-epigalloCatechin (EGC)) from green tea were evaluated for their ability to inhibit influenza virus replication in cell culture and for potentially direct virucidal effect.
|
| Animal Research | Catechin ameliorates cardiac dysfunction in rats with chronic heart failure by regulating the balance between Th17 and Treg cells.[Pubmed: 24760105]Inflamm Res. 2014 Aug;63(8):619-28.Disequilibrium of the cytokine network was reported to play an important role in the progression of chronic heart failure (CHF). Catechin exerts cardioprotection through treating many kinds of angiocardiopathy. However, the effects of Catechin on CHF are currently unclear. Therefore, the main aim of this study was to investigate the efficacy of Catechin on CHF rats as well as its relationship to immunoregulation.
|
| Structure Identification | J Agric Food Chem. 2014 Oct 15;62(41):10170-80.Plasticized poly(lactic acid)-poly(hydroxybutyrate) (PLA-PHB) blends incorporated with catechin intended for active food-packaging applications.[Pubmed: 25255375]
Bioorg Med Chem Lett. 2014 Jun 1;24(11):2582-4.Synthesis and radical-scavenging activity of a dimethyl catechin analogue.[Pubmed: 24792463]Catechin analogue 1 with methyl substituents ortho to the catechol hydroxyl groups was synthesized to improve the antioxidant ability of (+)-Catechin. The synthetic scheme involved a solid acid catalyzed Friedel-Crafts coupling of a cinnamyl alcohol derivative to 3,5-dibenzyloxyphenol followed by hydroxylation and then cyclization through an intermediate orthoester. The antioxidative radical scavenging activity of 1 against galvinoxyl radical, an oxyl radical, was found to be 28-fold more potent than (+)-Catechin. |

Catechin Dilution Calculator

Catechin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4447 mL | 17.2236 mL | 34.4471 mL | 68.8942 mL | 86.1178 mL |
| 5 mM | 0.6889 mL | 3.4447 mL | 6.8894 mL | 13.7788 mL | 17.2236 mL |
| 10 mM | 0.3445 mL | 1.7224 mL | 3.4447 mL | 6.8894 mL | 8.6118 mL |
| 50 mM | 0.0689 mL | 0.3445 mL | 0.6889 mL | 1.3779 mL | 1.7224 mL |
| 100 mM | 0.0344 mL | 0.1722 mL | 0.3445 mL | 0.6889 mL | 0.8612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Catechin inhibits cyclooxygenase-1 (COX-1) with an IC50 of 1.4 μM.
In Vitro:Catechin exhibits >95% inhibitory activity at 70 μg/mL against cyclooxygenase-1 (COX-1) with an IC50 of 1.4 μM[1]. A dose-dependent reduction in color is observed after 24 hours of treatment with Catechin, and 54.76% of the cells are dead at the highest concentration of Catechin tested (160 μg/mL) whereas the IC50 of Catechin is achieved at 127.62 μg/mL Catechin. A dose- and time-dependent increase in the induction of apoptosis is observed when MCF-7 cells are treated with Catechin. When compare to the control cells at 24 hours, 40.7 and 41.16% of the cells treated with 150 μg/mL and 300 μg/mL Catechin, respectively, undergo apoptosis. The expression levels of Caspase-3, -8, and -9 and p53 in MCF-7 cells treated with 150 μg/mL Catechin for 24 h increase by 5.81, 1.42, 3.29, and 2.68 fold, respectively, as compare to the levels in untreated control cells[2].
In Vivo:Animals treated with Catechin at the lowest tested dose, i.e., 50 mg/kg, p.o. have spent comparatively more time in exploring the novel object in the choice trial, however, the difference is not statistically significant. Catechin prevents the time-induced episodic memory deficits in a dose-dependent manner, the most effective being 200 mg/kg, p.o.. Treatment with Catechin prevents the rise in MPO level compare to DOX alone treatment group (21.98±9.44 and 36.76±4.39% in the hippocampus and the frontal cortex respectively)[3].
References:
[1]. Waffo-Téguo P, et al. Potential cancer-chemopreventive activities of wine stilbenoids and flavans extracted from grape (Vitis vinifera) cell cultures. Nutr Cancer. 2001;40(2):173-9.
[2]. Alshatwi AA. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J Exp Clin Cancer Res. 2010 Dec 17;29:167.
[3]. Cheruku SP, et al. Catechin ameliorates doxorubicin-induced neuronal cytotoxicity in in vitro and episodic memory deficit in in vivo in Wistar rats. Cytotechnology. 2018 Feb;70(1):245-259.
- Lincomycin
Catalog No.:BCC9010
CAS No.:154-21-2
- 2-Deoxy-D-glucose
Catalog No.:BCC4048
CAS No.:154-17-6
- ANQ 11125
Catalog No.:BCC6359
CAS No.:153966-48-4
- p-Hydroxyphenethyl vanillate
Catalog No.:BCN7555
CAS No.:1539303-03-1
- NBOH-2C-CN hydrochloride
Catalog No.:BCC8025
CAS No.:1539266-32-4
- Boc-Gln-ONp
Catalog No.:BCC3383
CAS No.:15387-45-8
- BLU9931
Catalog No.:BCC3979
CAS No.:1538604-68-0
- Fmoc-Trp-ol
Catalog No.:BCC2573
CAS No.:153815-60-2
- Dioxopromethazine hydrochloride
Catalog No.:BCC8946
CAS No.:15374-15-9
- Eriodictyol-8-glucoside
Catalog No.:BCN8029
CAS No.:153733-96-1
- 9,10-Bis(3,5-dihydroxyphenyl)anthracene
Catalog No.:BCC8793
CAS No.:153715-08-3
- H-Ile-OEt.HCl
Catalog No.:BCC2961
CAS No.:15366-32-3
- Thioguanine
Catalog No.:BCC2220
CAS No.:154-42-7
- 1,5-Anhydro-D-glucitol
Catalog No.:BCN2234
CAS No.:154-58-5
- Primverin
Catalog No.:BCC8238
CAS No.:154-60-9
- Primulaverin
Catalog No.:BCC8235
CAS No.:154-61-0
- Tripelennamine HCl
Catalog No.:BCC4523
CAS No.:154-69-8
- Bz-Arg-OH
Catalog No.:BCC2856
CAS No.:154-92-7
- Carmustine
Catalog No.:BCC5244
CAS No.:154-93-8
- Ipecoside
Catalog No.:BCN8301
CAS No.:15401-60-2
- Berberrubine
Catalog No.:BCN2651
CAS No.:15401-69-1
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications.[Pubmed:21827739]
Biochem Pharmacol. 2011 Dec 15;82(12):1807-21.
An expanding body of preclinical evidence suggests EGCG, the major Catechin found in green tea (Camellia sinensis), has the potential to impact a variety of human diseases. Apparently, EGCG functions as a powerful antioxidant, preventing oxidative damage in healthy cells, but also as an antiangiogenic and antitumor agent and as a modulator of tumor cell response to chemotherapy. Much of the cancer chemopreventive properties of green tea are mediated by EGCG that induces apoptosis and promotes cell growth arrest by altering the expression of cell cycle regulatory proteins, activating killer caspases, and suppressing oncogenic transcription factors and pluripotency maintain factors. In vitro studies have demonstrated that EGCG blocks carcinogenesis by affecting a wide array of signal transduction pathways including JAK/STAT, MAPK, PI3K/AKT, Wnt and Notch. EGCG stimulates telomere fragmentation through inhibiting telomerase activity. Various clinical studies have revealed that treatment by EGCG inhibits tumor incidence and multiplicity in different organ sites such as liver, stomach, skin, lung, mammary gland and colon. Recent work demonstrated that EGCG reduced DNMTs, proteases, and DHFR activities, which would affect transcription of TSGs and protein synthesis. EGCG has great potential in cancer prevention because of its safety, low cost and bioavailability. In this review, we discuss its cancer preventive properties and its mechanism of action at numerous points regulating cancer cell growth, survival, angiogenesis and metastasis. Therefore, non-toxic natural agent could be useful either alone or in combination with conventional therapeutics for the prevention of tumor progression and/or treatment of human malignancies.
Plasticized poly(lactic acid)-poly(hydroxybutyrate) (PLA-PHB) blends incorporated with catechin intended for active food-packaging applications.[Pubmed:25255375]
J Agric Food Chem. 2014 Oct 15;62(41):10170-80.
Active biobased packaging materials based on poly(lactic acid)-poly(hydroxybutyrate) (PLA-PHB) blends were prepared by melt blending and fully characterized. Catechin incorporation, as antioxidant compound, enhanced the thermal stability, whereas its release was improved by the addition of acetyl(tributyl citrate) (ATBC) as plasticizer. Whereas the incorporation of ATBC resulted in a reduction of elastic modulus and hardness, Catechin addition produced more rigid materials due to hydrogen-bonding interactions between Catechin hydroxyl groups and carbonyl groups of PLA and PHB. The quantification of Catechin released into a fatty food simulant and the antioxidant effectiveness after the release process were demonstrated. The effect of the materials' exposure to a food simulant was also investigated. PHB-added materials maintained their structural and mechanical properties after 10 days in a test medium that represents the worst foreseeable conditions of the intended use. Thus, plasticized PLA-PHB blends with Catechin show their potential as biobased active packaging for fatty food.
Antiviral effect of catechins in green tea on influenza virus.[Pubmed:16137775]
Antiviral Res. 2005 Nov;68(2):66-74.
Polyphenolic compound Catechins ((-)-epigalloCatechin gallate (EGCG), (-)-epiCatechin gallate (ECG) and (-)-epigalloCatechin (EGC)) from green tea were evaluated for their ability to inhibit influenza virus replication in cell culture and for potentially direct virucidal effect. Among the test compounds, the EGCG and ECG were found to be potent inhibitors of influenza virus replication in MDCK cell culture and this effect was observed in all influenza virus subtypes tested, including A/H1N1, A/H3N2 and B virus. The 50% effective inhibition concentration (EC50) of EGCG, ECG, and EGC for influenza A virus were 22-28, 22-40 and 309-318 microM, respectively. EGCG and ECG exhibited hemagglutination inhibition activity, EGCG being more effective. However, the sensitivity in hemagglutination inhibition was widely different among three different subtypes of influenza viruses tested. Quantitative RT-PCR analysis revealed that, at high concentration, EGCG and ECG also suppressed viral RNA synthesis in MDCK cells whereas EGC failed to show similar effect. Similarly, EGCG and ECG inhibited the neuraminidase activity more effectively than the EGC. The results show that the 3-galloyl group of Catechin skeleton plays an important role on the observed antiviral activity, whereas the 5'-OH at the trihydroxy benzyl moiety at 2-position plays a minor role. The results, along with the HA type-specific effect, suggest that the antiviral effect of Catechins on influenza virus is mediated not only by specific interaction with HA, but altering the physical properties of viral membrane.
Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study.[Pubmed:11470725]
Am J Clin Nutr. 2001 Aug;74(2):227-32.
BACKGROUND: Epidemiologic studies suggest that tea consumption may reduce the risk of cardiovascular diseases, but results are inconsistent. Catechins, which belong to the flavonoid family, are the main components of tea and may be responsible for the alleged protective effect. Taking Catechin sources other than tea into account might clarify the reported associations. OBJECTIVE: The objective was to evaluate the association between Catechin intake and the incidence of and mortality from ischemic heart disease and stroke. DESIGN: We evaluated the effect of a high Catechin intake by using data from the Zutphen Elderly Study, a prospective cohort study of 806 men aged 65-84 y at baseline in 1985. RESULTS: The mean (+/-SD) Catechin intake at baseline was 72 +/- 47.8 mg, mainly from black tea, apples, and chocolate. A total of 90 deaths from ischemic heart disease were documented. Catechin intake was inversely associated with ischemic heart disease mortality; the multivariate-adjusted risk ratio in the highest tertile of intake was 0.49 (95% CI: 0.27, 0.88; P for trend: 0.017). After multivariate adjustment, Catechin intake was not associated with the incidence of myocardial infarction (risk ratio in the highest tertile of intake: 0.70; 95% CI: 0.39, 1.26; P for trend: 0.232). After adjustment for tea consumption and flavonol intake, a 7.5-mg increase in Catechin intake from sources other than tea was associated with a tendency for a 20% reduction in ischemic heart disease mortality risk (P = 0.114). There was no association between Catechin intake and stroke incidence or mortality. CONCLUSION: Catechins, whether from tea or other sources, may reduce the risk of ischemic heart disease mortality but not of stroke.
Effect of emulsification on the skin permeation and UV protection of catechin.[Pubmed:23639253]
Pharm Dev Technol. 2014 Jun;19(4):395-400.
An anti-aging effect may be obtained by skin application of tea Catechins (Camellia sinensis) since they have high ultraviolet (UV)-protection activity. In this study, the skin permeation of Catechin (C), epiCatechin (EC), epigalloCatechin (EGC), epiCatechin gallate (ECg) and epigalloCatechin gallate (EGCg) was determined and compared, and the effect of emulsification on the skin permeation of C was measured. The UV-protective effect of C was also determined. The in vitro skin permeability of each Catechin derivative was determined using side-by-side diffusion of cells. The UV-protective effect of C was determined by applying different concentrations of C to the solution or emulsion on a three-dimensional cultured human skin model or normal human epidermal keratinocytes with UV-irradiation. ECg and EGCg with gallate groups showed lower skin permeability than C, EC and EGC without gallate groups, suggesting that the skin permeability of Catechin derivatives may be dependent on the existence of a gallate group. Interestingly, the skin permeation of C was increased by an o/w emulsification. In addition, the C emulsion showed a significantly higher UV-protective effect by C than that with its aqueous solution. These results suggest that the o/w emulsion of Catechin derivatives is probably useful as a cosmetic formulation with anti-aging efficacy.
Catechin ameliorates cardiac dysfunction in rats with chronic heart failure by regulating the balance between Th17 and Treg cells.[Pubmed:24760105]
Inflamm Res. 2014 Aug;63(8):619-28.
OBJECTIVE: Disequilibrium of the cytokine network was reported to play an important role in the progression of chronic heart failure (CHF). Catechin exerts cardioprotection through treating many kinds of angiocardiopathy. However, the effects of Catechin on CHF are currently unclear. Therefore, the main aim of this study was to investigate the efficacy of Catechin on CHF rats as well as its relationship to immunoregulation. METHODS: CHF was induced in rats by ligation of the abdominal aorta. Myocardial function was evaluated by left ventricular systolic pressure and left ventricular end-diastolic pressure. The cytokine level was measured by enzyme-linked immunosorbent assay. Th17 and Treg levels in peripheral blood and spleen were analyzed by flow cytometry. RESULTS: The results showed that Catechin treatment (50, 100 mg/kg/day) markedly improved myocardial function in rats treated with abdominal aortic coarctation. Severity of myocardial dysfunction in CHF rats significantly correlated with serum values of interleukin-17 (IL-17)/IL-10. Further results indicated Catechin obviously inhibited immune activation, regulated unbalanced levels of IL-17/IL-10, and reversed abnormal polarization of TH17 as well as Treg in peripheral blood and spleen. CONCLUSIONS: Taken together, oral administration of Catechin effectively suppressed abdominal aorta ligation-induced CHF in rats, which was closely associated with its modulation on Th17 and Treg.
Synthesis and radical-scavenging activity of a dimethyl catechin analogue.[Pubmed:24792463]
Bioorg Med Chem Lett. 2014 Jun 1;24(11):2582-4.
Catechin analogue 1 with methyl substituents ortho to the catechol hydroxyl groups was synthesized to improve the antioxidant ability of (+)-Catechin. The synthetic scheme involved a solid acid catalyzed Friedel-Crafts coupling of a cinnamyl alcohol derivative to 3,5-dibenzyloxyphenol followed by hydroxylation and then cyclization through an intermediate orthoester. The antioxidative radical scavenging activity of 1 against galvinoxyl radical, an oxyl radical, was found to be 28-fold more potent than (+)-Catechin.


