Fuscaxanthone CCAS# 15404-76-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15404-76-9 | SDF | Download SDF |
| PubChem ID | 231412 | Appearance | Yellow powder |
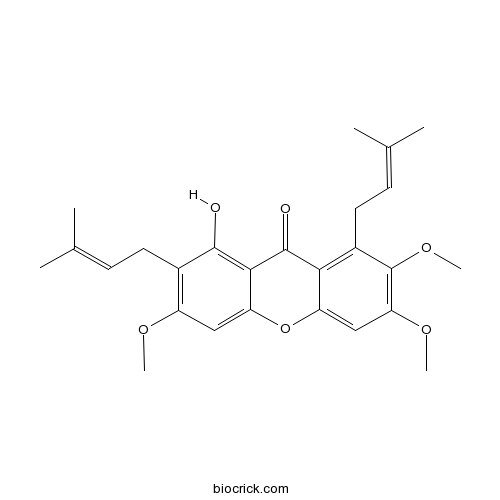
| Formula | C26H30O6 | M.Wt | 438.5 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Synonyms | 3,6-Dimethylmangostin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-hydroxy-3,6,7-trimethoxy-2,8-bis(3-methylbut-2-enyl)xanthen-9-one | ||
| SMILES | CC(=CCC1=C(C=C2C(=C1O)C(=O)C3=C(C(=C(C=C3O2)OC)OC)CC=C(C)C)OC)C | ||
| Standard InChIKey | FCIKYGUVHWZSJV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H30O6/c1-14(2)8-10-16-18(29-5)12-20-23(24(16)27)25(28)22-17(11-9-15(3)4)26(31-7)21(30-6)13-19(22)32-20/h8-9,12-13,27H,10-11H2,1-7H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fuscaxanthone C is a natural product from Garcinia mangostana. |
| In vitro | Cytotoxic constituents from Cratoxylum arborescens.[Pubmed: 15729629 ]Planta Med. 2005 Feb;71(2):181-3.A new natural xanthone, 1,3-dihydroxy-6,7-dimethoxy-2,8-diprenylxanthone, together with four known compounds, Fuscaxanthone C, 1,7-dihydroxyxanthone, 3-geranyloxy-6-methyl-1,8-dihydroxyanthraquinone and 2-geranylemodin were isolated from the stem bark of Cratoxylum arborescens (Vahl) Blume.
α-mangostin and β-mangostin from Cratoxylum glaucum[Reference: WebLink]Research Journal of Chemistry & Environment, 2011 , 15 (2) :62-66.Our continuing interest in xanthones and anthraquinones from the Cratoxylum genus has led us to look at Cratoxylum glaucum. This resulted in the isolation of α-mangostin (1), β-mangostin (2), Fuscaxanthone C (3), 3-geranyloxy-6-methyl-l, 8-dihydroxyanthraquinone (4), β-sitosterol (5), 1, 8-dihydroxy-3-methoxy-6-methylanthraquinone (6), stigmasterol (7), friedelin (8) and betulinic acid (9).
|

Fuscaxanthone C Dilution Calculator

Fuscaxanthone C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2805 mL | 11.4025 mL | 22.805 mL | 45.61 mL | 57.0125 mL |
| 5 mM | 0.4561 mL | 2.2805 mL | 4.561 mL | 9.122 mL | 11.4025 mL |
| 10 mM | 0.2281 mL | 1.1403 mL | 2.2805 mL | 4.561 mL | 5.7013 mL |
| 50 mM | 0.0456 mL | 0.2281 mL | 0.4561 mL | 0.9122 mL | 1.1403 mL |
| 100 mM | 0.0228 mL | 0.114 mL | 0.2281 mL | 0.4561 mL | 0.5701 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Berberrubine
Catalog No.:BCN2651
CAS No.:15401-69-1
- Ipecoside
Catalog No.:BCN8301
CAS No.:15401-60-2
- Carmustine
Catalog No.:BCC5244
CAS No.:154-93-8
- Bz-Arg-OH
Catalog No.:BCC2856
CAS No.:154-92-7
- Tripelennamine HCl
Catalog No.:BCC4523
CAS No.:154-69-8
- Primulaverin
Catalog No.:BCC8235
CAS No.:154-61-0
- Primverin
Catalog No.:BCC8238
CAS No.:154-60-9
- 1,5-Anhydro-D-glucitol
Catalog No.:BCN2234
CAS No.:154-58-5
- Thioguanine
Catalog No.:BCC2220
CAS No.:154-42-7
- Catechin
Catalog No.:BCN1688
CAS No.:154-23-4
- Lincomycin
Catalog No.:BCC9010
CAS No.:154-21-2
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
Cytotoxic constituents from Cratoxylum arborescens.[Pubmed:15729629]
Planta Med. 2005 Feb;71(2):181-3.
A new natural xanthone, 1,3-dihydroxy-6,7-dimethoxy-2,8-diprenylxanthone, together with four known compounds, Fuscaxanthone C, 1,7-dihydroxyxanthone, 3-geranyloxy-6-methyl-1,8-dihydroxyanthraquinone and 2-geranylemodin were isolated from the stem bark of Cratoxylum arborescens (Vahl) Blume. The structure elucidations were achieved through spectroscopic analyses. Compounds 1 and 5 showed moderate activity against the human small cell lung cancer NCI-H187 cell line with the IC50 values of 3.69 +/- 1.27 microg/mL and 3.08 +/- 0.73 microg/mL, respectively.


