IpecosideCAS# 15401-60-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15401-60-2 | SDF | Download SDF |
| PubChem ID | 442249 | Appearance | Powder |
| Formula | C27H35NO12 | M.Wt | 565.5663 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
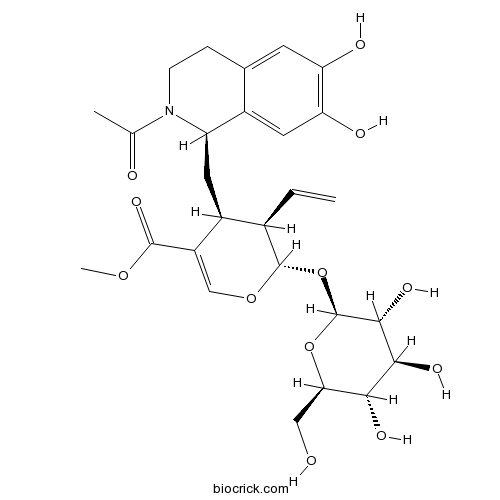
| Chemical Name | methyl (2S,3R,4S)-4-[[(1R)-2-acetyl-6,7-dihydroxy-3,4-dihydro-1H-isoquinolin-1-yl]methyl]-3-ethenyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,4-dihydro-2H-pyran-5-carboxylate | ||
| SMILES | CC(=O)N1CCC2=CC(=C(C=C2C1CC3C(C(OC=C3C(=O)OC)OC4C(C(C(C(O4)CO)O)O)O)C=C)O)O | ||
| Standard InChIKey | QISXROCIXKXUPS-OWVLCBNUSA-N | ||
| Standard InChI | InChI=1S/C27H35NO12/c1-4-14-16(8-18-15-9-20(32)19(31)7-13(15)5-6-28(18)12(2)30)17(25(36)37-3)11-38-26(14)40-27-24(35)23(34)22(33)21(10-29)39-27/h4,7,9,11,14,16,18,21-24,26-27,29,31-35H,1,5-6,8,10H2,2-3H3/t14-,16+,18-,21-,22-,23+,24-,26+,27+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | J Biol Chem. 2008 Dec 12;283(50):34650-9.The new beta-D-glucosidase in terpenoid-isoquinoline alkaloid biosynthesis in Psychotria ipecacuanha.[Pubmed: 18927081]Ipecac alkaloids produced in the medicinal plant Psychotria ipecacuanha such as emetine and cephaeline possess a monoterpenoid-tetrahydroisoquinoline skeleton, which is formed by condensation of dopamine and secologanin. Deglucosylation of one of the condensed products N-deacetylisoIpecoside (1 alpha(S)-epimer) is considered to be a part of the reactions for emetine biosynthesis, whereas its 1 beta(R)-epimer N-deacetylIpecoside is converted to Ipecoside in P. ipecacuanha.
|

Ipecoside Dilution Calculator

Ipecoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7681 mL | 8.8407 mL | 17.6814 mL | 35.3628 mL | 44.2035 mL |
| 5 mM | 0.3536 mL | 1.7681 mL | 3.5363 mL | 7.0726 mL | 8.8407 mL |
| 10 mM | 0.1768 mL | 0.8841 mL | 1.7681 mL | 3.5363 mL | 4.4203 mL |
| 50 mM | 0.0354 mL | 0.1768 mL | 0.3536 mL | 0.7073 mL | 0.8841 mL |
| 100 mM | 0.0177 mL | 0.0884 mL | 0.1768 mL | 0.3536 mL | 0.442 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Carmustine
Catalog No.:BCC5244
CAS No.:154-93-8
- Bz-Arg-OH
Catalog No.:BCC2856
CAS No.:154-92-7
- Tripelennamine HCl
Catalog No.:BCC4523
CAS No.:154-69-8
- Primulaverin
Catalog No.:BCC8235
CAS No.:154-61-0
- Primverin
Catalog No.:BCC8238
CAS No.:154-60-9
- 1,5-Anhydro-D-glucitol
Catalog No.:BCN2234
CAS No.:154-58-5
- Thioguanine
Catalog No.:BCC2220
CAS No.:154-42-7
- Catechin
Catalog No.:BCN1688
CAS No.:154-23-4
- Lincomycin
Catalog No.:BCC9010
CAS No.:154-21-2
- 2-Deoxy-D-glucose
Catalog No.:BCC4048
CAS No.:154-17-6
- ANQ 11125
Catalog No.:BCC6359
CAS No.:153966-48-4
- p-Hydroxyphenethyl vanillate
Catalog No.:BCN7555
CAS No.:1539303-03-1
- Berberrubine
Catalog No.:BCN2651
CAS No.:15401-69-1
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
The new beta-D-glucosidase in terpenoid-isoquinoline alkaloid biosynthesis in Psychotria ipecacuanha.[Pubmed:18927081]
J Biol Chem. 2008 Dec 12;283(50):34650-9.
Ipecac alkaloids produced in the medicinal plant Psychotria ipecacuanha such as emetine and cephaeline possess a monoterpenoid-tetrahydroisoquinoline skeleton, which is formed by condensation of dopamine and secologanin. Deglucosylation of one of the condensed products N-deacetylisoIpecoside (1 alpha(S)-epimer) is considered to be a part of the reactions for emetine biosynthesis, whereas its 1 beta(R)-epimer N-deacetylIpecoside is converted to Ipecoside in P. ipecacuanha. Here, we isolated a cDNA clone Ipeglu1 encoding Ipecac alkaloid beta-D-glucosidase from P. ipecacuanha. The deduced protein showed 54 and 48% identities to raucaffricine beta-glucosidase and strictosidine beta-glucosidase, respectively. Recombinant IpeGlu1 enzyme preferentially hydrolyzed glucosidic Ipecac alkaloids except for their lactams, but showed poor or no activity toward other substrates, including terpenoid-indole alkaloid glucosides. Liquid chromatography-tandem mass spectrometry analysis of deglucosylated products of N-deacetylisoIpecoside revealed spontaneous transitions of the highly reactive aglycons, one of which was supposed to be the intermediate for emetine biosynthesis. IpeGlu1 activity was extremely poor toward 7-O-methyl and 6,7-O,O-dimethyl derivatives. However, 6-O-methyl derivatives were hydrolyzed as efficiently as non-methylated substrates, suggesting the possibility of 6-O-methylation prior to deglucosylation by IpeGlu1. In contrast to the strictosidine beta-glucosidase that stereospecifically hydrolyzes 3 alpha(S)-epimer in terpenoid-indole alkaloid biosynthesis, IpeGlu1 lacked stereospecificity for its substrates where 1 beta(R)-epimers were preferred to 1 alpha(S)-epimers, although Ipecoside (1 beta(R)) is a major alkaloidal glucoside in P. ipecacuanha, suggesting the compartmentalization of IpeGlu1 from Ipecoside. These facts have significant implications for distinct physiological roles of 1 alpha(S)- and 1 beta(R)-epimers and for the involvement of IpeGlu1 in the metabolic fate of both of them.


