MarimastatMMPs inhibitor,board spectrum CAS# 154039-60-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154039-60-8 | SDF | Download SDF |
| PubChem ID | 119031 | Appearance | Powder |
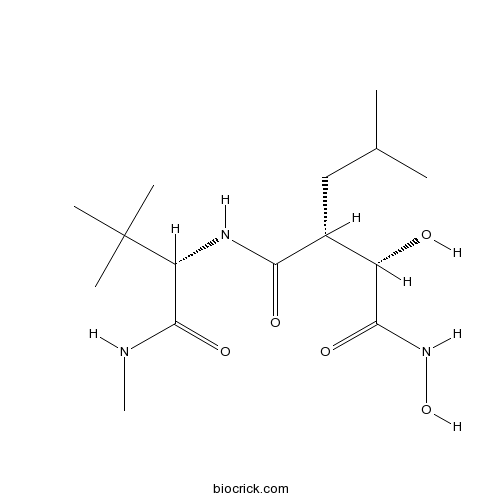
| Formula | C15H29N3O5 | M.Wt | 331.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BB 2516, Marimistat | ||
| Solubility | DMSO : 100 mg/mL (301.74 mM; Need ultrasonic) | ||
| Chemical Name | (2R,3S)-N-[(2S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl]-N',3-dihydroxy-2-(2-methylpropyl)butanediamide | ||
| SMILES | CC(C)CC(C(C(=O)NO)O)C(=O)NC(C(=O)NC)C(C)(C)C | ||
| Standard InChIKey | OCSMOTCMPXTDND-OUAUKWLOSA-N | ||
| Standard InChI | InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Broad spectrum inhibitor of MMPs (IC50 values are 3, 5, 6, 9 and 13 nM for MMP-9, MMP-1, MMP-2, MMP-14 and MMP-7 respectively). Inhibits peritoneal dissemination of human gastric cancer cells in vivo through inhibition of tumor angiogenesis. |

Marimastat Dilution Calculator

Marimastat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0174 mL | 15.0871 mL | 30.1741 mL | 60.3482 mL | 75.4353 mL |
| 5 mM | 0.6035 mL | 3.0174 mL | 6.0348 mL | 12.0696 mL | 15.0871 mL |
| 10 mM | 0.3017 mL | 1.5087 mL | 3.0174 mL | 6.0348 mL | 7.5435 mL |
| 50 mM | 0.0603 mL | 0.3017 mL | 0.6035 mL | 1.207 mL | 1.5087 mL |
| 100 mM | 0.0302 mL | 0.1509 mL | 0.3017 mL | 0.6035 mL | 0.7544 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Marimastat is a broad range inhibitor of Matrix metalloproteinases with IC50 values of 5, 6, 13, 3 and 9 nM for MMP-1, MMP-2, MMP-7 , MMP-9 and MMP-14 [1].
Marimastat is an orally bioavailable inhibitor with an absolute bioavailability of 20%–50% in preclinical studies, which can bind covalently to the zinc atom of the MMP-active site by a collagen-mimicking hydroxamate structure [1].
A reduction in the size and number of metastatic foci in treated compared with the control animals was demonstrated by experimental metastases models against lung and breast cancer. The studies were carried out at doses of 100–500 mg/kg per day, and the agent induced gastrointestinal toxicity and weight loss, as well as hemorrhage, fibrosis, inflammation, and necrosis at particular ankle and knee tissues. Single oral doses of up to 800 mg were well tolerated and did not lead to obvious toxicity. Peak plasma concentrations can be detected within 1.5–3 hours after oral administration, and the elimination half-life was estimated as a range of 8–10 hours. No plasma accumulation was detected after an oral doses of 50–200 mg in continuous administration twice a day for 6 consecutive days [2,3].
Pharmacological studies demonstrated that marimastat is well absorbed from the gastrointestinal tract and exhibits a linear pharmacokinetic behavior. The minimum plasma concentration was found after exceeding 10 mg doses twice a day, which were sixfold greater than the required for inhibition of MMP in vitro. Complain to the patients treated with gemcitabine, the most effective chemotherapeutic agent against the nonmetastatic pancreatic cancer, the patients who received high doses of marimastat had a 1-year survival rates. It is encouraging that the patients with unresectable gastric cancer who were treated with marimastat show a modest increase in survival [1,2].
References:
1.Hidalgo M, Eckhardt SG, Development of matrix metalloproteinase inhibitors in cancer therapy. JOURNAL OF THE NATIONAL CANCER INSTITUTE. 2001, 93(3):178-193.
2.Coussens LM, Fingleton B , Matrisian LM , Cancer therapy - Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. SCIENCE, 295 (5564): 2387-2392.
3.Van Wijngaarden J , Snoeks TJA , van Beek E, et al . An in vitro model that can distinguish between effects on angiogenesis and on established vasculature: Actions of TNP-470, marimastat and the tubulin-binding agent Ang-510. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, 2000, 391 (2): 1161-1165.
- Berberrubine
Catalog No.:BCN2651
CAS No.:15401-69-1
- Ipecoside
Catalog No.:BCN8301
CAS No.:15401-60-2
- Carmustine
Catalog No.:BCC5244
CAS No.:154-93-8
- Bz-Arg-OH
Catalog No.:BCC2856
CAS No.:154-92-7
- Tripelennamine HCl
Catalog No.:BCC4523
CAS No.:154-69-8
- Primulaverin
Catalog No.:BCC8235
CAS No.:154-61-0
- Primverin
Catalog No.:BCC8238
CAS No.:154-60-9
- 1,5-Anhydro-D-glucitol
Catalog No.:BCN2234
CAS No.:154-58-5
- Thioguanine
Catalog No.:BCC2220
CAS No.:154-42-7
- Catechin
Catalog No.:BCN1688
CAS No.:154-23-4
- Lincomycin
Catalog No.:BCC9010
CAS No.:154-21-2
- 2-Deoxy-D-glucose
Catalog No.:BCC4048
CAS No.:154-17-6
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
Incorporation of Bulky and Cationic Cyclam-Triazole Moieties into Marimastat Can Generate Potent MMP Inhibitory Activity without Inducing Cytotoxicity.[Pubmed:24551546]
ChemistryOpen. 2013 Jun;2(3):99-105.
The synthesis and matrix metalloproteinase (MMP) inhibitory activity of a cyclam-Marimastat conjugate and its metal complexes are described. The conjugate, synthesized with a copper(I)-catalyzed Huisgen 1,3-dipolar cycloaddition ("click" reaction), contains two zinc-binding groups (ZBGs). The metal complexation behavior with copper(II) and zinc(II) was investigated using UV/Vis spectrophotometry and (1)H NMR spectroscopy, respectively, demonstrating that the first equivalent of the metal ion was chelated by the cyclam-triazole moiety rather than the hydroxamic acid site. Thus, the corresponding mononuclear metal-cyclam complexes were successfully prepared with one equivalent of the metal salt. Both the cyclam-Marimastat conjugate and its metal complexes exhibited slightly reduced potency against MMP-1, but essentially identical inhibitory activity against MMP-3. The conjugate and its metal complexes displayed little or no cytotoxicity, further supporting their potential suitability for imaging MMP localization and activity. To the best of our knowledge, this is the first report that describes the incorporation of metal complexes into an MMP inhibitor without influencing the preexisting ZBG, and the first report of the evaluation of structures containing more than one ZBG as MMP inhibitors.
Intraosseous infusion of the distal phalanx compared to systemic intravenous infusion for marimastat delivery to equine lamellar tissue.[Pubmed:26073286]
Vet J. 2015 Sep;205(3):357-63.
No validated laminitis drug therapy exists, yet pharmaceutical agents with potential for laminitis prevention have been identified. Many of these are impractical for systemic administration but may be effective if administered locally. This study compared intraosseous infusion of the distal phalanx (IOIDP) with systemic intravenous constant rate infusion (CRI) to determine which was more effective for lamellar Marimastat delivery. Ultrafiltration probes were placed in both forefeet of five horses to collect lamellar interstitial fluid as lamellar ultrafiltrate (LUF). Marimastat solution (3.5 mg/mL) containing lidocaine (20 mg/mL) was infused by IOIDP at 0.15 mL/min for 12 h. After a 12 h wash-out, Marimastat (3.5 mg/mL) and lidocaine were infused by constant rate infusion (CRI) at 0.15 mL/min for 12 h. LUF, plasma and lamellar tissue Marimastat concentrations were quantified using UPLC-MS. Zymography was used to establish the inhibitory concentrations of Marimastat for equine lamellar matrix metalloproteinases (MMPs). Data were analysed non-parametrically. There was no difference between the steady-state Marimastat concentration in lamellar ultrafiltrate (LUF[M]) during IOIDP (139[88-497] ng/mL) and CRI (136[93-157] ng/mL). During IOIDP, there was no difference between Marimastat concentrations in the treated foot (139[88-497] ng/mL), the untreated foot (91[63-154] ng/mL) and plasma (101[93-118] ng/mL). LUF[M] after IOIDP and CRI were >IC50 of lamellar MMP-2 and 9, but below the concentration considered necessary for in vivo laminitis prevention. Lamellar drug delivery during IOIDP was inconsistent and did not achieve higher lamellar Marimastat concentrations than CRI. Modification or refinement of the IOIDP technique is necessary if it is to be consistently effective.
Regional intravenous limb perfusion compared to systemic intravenous administration for marimastat delivery to equine lamellar tissue.[Pubmed:25641095]
J Vet Pharmacol Ther. 2015 Aug;38(4):392-9.
Pharmaceutical agents with potential for laminitis prevention have been identified. Many of these, including the MMP inhibitor Marimastat, are impractical for systemic administration. This study compared local delivery of Marimastat by regional limb perfusion (RLP) to systemic intravenous bolus dosing (SIVB), and established whether RLP results in local lamellar drug delivery. Six adult horses received 0.23 mg/kg of Marimastat by RLP followed by 0.23 mg/kg Marimastat by SIVB, with a 24-h washout period. Lamellar ultrafiltration probes sampled lamellar interstitial fluid as lamellar ultrafiltrate (LUF). LUF and plasma Marimastat concentrations (LUF[M] and P[M] respectively) were measured for 24 h after each treatment. Regional pharmacokinetic parameters were calculated using noncompartmental analyses. The LUF C(max) following RLP was 232 [34-457] times that following SIVB. LUF[M] after RLP were higher than those obtained after SIVB for 18 h (P < 0.03). Median LUF[M] were > IC(90) of equine lamellar MMP-2 and MMP-9 for 9 h after tourniquet removal. RLP appeared superior to SIVB for lamellar Marimastat delivery (higher LUF C(max),, AUC and T > IC(90) of lamellar MMPs). However, frequent dosing is necessary to achieve therapeutic lamellar concentrations. RLP could be used to investigate whether Marimastat prevents experimentally induced laminitis. Further refinement of the technique and dosing interval is necessary before clinical application.
The matrix metalloproteinase inhibitor marimastat promotes neural progenitor cell differentiation into neurons by gelatinase-independent TIMP-2-dependent mechanisms.[Pubmed:23098139]
Stem Cells Dev. 2013 Feb 1;22(3):345-58.
Metalloproteinases (MMPs) and their endogenous inhibitors (TIMPs), produced in the brain by cells of non-neural and neural origin, including neural progenitors (NPs), are emerging as regulators of nervous system development and adult brain functions. In the present study, we explored whether MMP-2, MMP-9, and TIMP-2, abundantly produced in the brain, modulate NP developmental properties. We found that treatment of NPs, isolated from the murine fetal cerebral cortex or adult subventricular zone, with the clinically tested broad-spectrum MMP inhibitor Marimastat profoundly affected the NP differentiation fate. Marimastat treatment allowed for an enrichment of our cultures in neuronal cells, inducing NPs to generate higher percentage of neurons and a lower percentage of astrocytes, possibly affecting NP commitment. Consistently with its proneurogenic effect, Marimastat early downregulated the expression of Notch target genes, such as Hes1 and Hes5. MMP-2 and MMP-9 profiling on proliferating and differentiating NPs revealed that MMP-9 was not expressed under these conditions, whereas MMP-2 increased in the medium as pro-MMP-2 (72 kDa) during differentiation; its active form (62 kDa) was not detectable by gel zymography. MMP-2 silencing or administration of recombinant active MMP-2 demonstrated that MMP-2 does not affect NP neuronal differentiation, nor it is involved in the Marimastat proneurogenic effect. We also found that TIMP-2 is expressed in NPs and increases during late differentiation, mainly as a consequence of astrocyte generation. Endogenous TIMP-2 did not modulate NP neurogenic potential; however, the proneurogenic action of Marimastat was mediated by TIMP-2, as demonstrated by silencing experiments. In conclusion, our data exclude a major involvement of MMP-2 and MMP-9 in the regulation of basal NP differentiation, but highlight the ability of TIMP-2 to act as key effector of the proneurogenic response to an inducing stimulus such as Marimastat.
Matrix metalloproteinases participate in osteosarcoma invasion.[Pubmed:16083752]
J Surg Res. 2005 Aug;127(2):151-6.
BACKGROUND: Osteosarcoma (OS) is a highly malignant bone tumor and is the most frequent malignant bone tumor in children and adolescents. Metastases are the major cause of death, and patients with relapse have poor prognosis. Several solid tumors display enhanced expression of matrix metalloproteinases (MMPs), and recently MMP-inhibitors have entered clinical trials. A disturbance of the MMP system in favor of enhanced proteolytic activity may be suspected in OS because OS growth is accompanied by both enhanced local bone destruction and bone formation, two processes that are dependant on proteolytic enzymes. Thus, the aim of the present study was to evaluate the involvement of MMPs in a panel of human OS cell lines, xenografts and biopsies. MATERIAL AND METHODS: Expression of MMPs and their endogenous inhibitors were studied by zymography and Northern blot analyses. In vitro invasion of OS cell lines and effects of MMP-inhibitors (Marimastat and doxycycline) were assessed in the transwell chamber assay. RESULTS: In vitro invasiveness was compared with gelatinase activity, and the most invasive cell line secreted the highest amounts of MMP-2 and MMP-9. Two different MMP-inhibitors significantly reduced OS cell invasion. The majority of the OS xenografts expressed both the inactive and active form of MMP-2 and in some cases also MMP-9. The biopsies from primary and metastatic OS also expressed MMP-2 mRNA. However, MMP-9 levels were higher in the biopsies than in the xenografts. CONCLUSION: The obtained results support the hypothesis that MMPs and their endogenous inhibitors participate in the invasive process of human OS.
Reduced angiogenesis in peritoneal dissemination of gastric cancer through gelatinase inhibition.[Pubmed:14524532]
Clin Exp Metastasis. 2003;20(5):431-5.
Marimastat is a broad-spectrum matrix metalloproteinase (MMP) inhibitor that inhibits almost all major MMPs, key enzymes in gastric cancer invasion and metastasis. We investigated the ability of Marimastat to inhibit tumor angiogenesis in the severe combined immuno-deficient (SCID) mouse/human gastric cancer model of peritoneal dissemination. A human stomach adenocarcinoma cell line, TMK-1, was injected intraperitoneally into SCID mice. On the 7th day after tumor inoculation, the administration of Marimastat (27 mg/kg/day) was initiated and the treatment was continued for 2 weeks using subcutaneously-inoculating mini-osmotic pumps. On the 21st day, the mice were killed and the disseminated nodules were evaluated. Total weights, numbers, and the microvascular density of the disseminating nodules were significantly lower in mice treated with Marimastat compared to the control group. Film in situ zymography demonstrated that net gelatinolytic activity in the tissues was weaker in treated-group nodules than in control-group nodules. Thus, our results suggested that Marimastat inhibited peritoneal dissemination of human gastric cancer cells through inhibition of tumor angiogenesis, possibly involving the down-regulation of gelatinases, in SCID mice injected with human gastric cancer cells.
Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat.[Pubmed:9364582]
Pharmacol Ther. 1997;75(1):69-75.
Matrix metalloproteinases (MMPs) are a homologous family of enzymes that are involved in tissue remodeling and morphogenesis. Collectively, these enzymes are capable of degrading all components of the extracellular matrix, and they play an important role in normal physiologic conditions, such as wound healing and other processes involving tissue remodeling. However, increased activity of these enzymes now has been observed in a number of different pathological conditions, and it has been hypothesized that such increased activity of MMPs might play a role in the pathogenesis of these conditions. Cancer is one such condition; extracellular matrices constitute the principal barrier to tumor growth and spread, and there is growing experimental evidence that malignant tumors utilize MMPs to overcome these barriers. Consequently, inhibitors of MMPs represent an attractive target for a new class of anticancer agents. Marimastat and batimastat are potent broad-spectrum inhibitors of all major MMPs and have been shown to prevent or reduce spread and growth of a number of different malignant tumors in numerous animal models. Both agents are now in advanced clinical testing in a number of different solid tumors in North America and Europe. The purpose of this paper is to review available preclinical and emerging clinical data, using batimastat and Marimastat as prototype MMP inhibitors in the cancer area.


