ARP 101MMP inhibitor,potent and selective CAS# 849773-63-3 |
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Ro 32-3555
Catalog No.:BCC2377
CAS No.:190648-49-8
- CP 471474
Catalog No.:BCC2373
CAS No.:210755-45-6
- SB-3CT
Catalog No.:BCC5486
CAS No.:292605-14-2
- NSC 405020
Catalog No.:BCC2120
CAS No.:7497-07-6
- ARP 101
Catalog No.:BCC2371
CAS No.:849773-63-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 849773-63-3 | SDF | Download SDF |
| PubChem ID | 11292680 | Appearance | Powder |
| Formula | C20H26N2O5S | M.Wt | 406.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol | ||
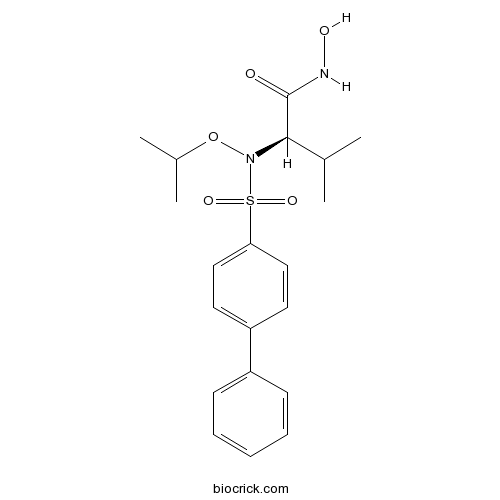
| Chemical Name | (2R)-N-hydroxy-3-methyl-2-[(4-phenylphenyl)sulfonyl-propan-2-yloxyamino]butanamide | ||
| SMILES | CC(C)C(C(=O)NO)N(OC(C)C)S(=O)(=O)C1=CC=C(C=C1)C2=CC=CC=C2 | ||
| Standard InChIKey | DGZZVIWCMGVHGV-LJQANCHMSA-N | ||
| Standard InChI | InChI=1S/C20H26N2O5S/c1-14(2)19(20(23)21-24)22(27-15(3)4)28(25,26)18-12-10-17(11-13-18)16-8-6-5-7-9-16/h5-15,19,24H,1-4H3,(H,21,23)/t19-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of MMP-2 that displays ~ 600-fold selectivity over MMP-1 (IC50 values are 0.81 and 486 nM respectively). |

ARP 101 Dilution Calculator

ARP 101 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.46 mL | 12.3001 mL | 24.6002 mL | 49.2005 mL | 61.5006 mL |
| 5 mM | 0.492 mL | 2.46 mL | 4.92 mL | 9.8401 mL | 12.3001 mL |
| 10 mM | 0.246 mL | 1.23 mL | 2.46 mL | 4.92 mL | 6.1501 mL |
| 50 mM | 0.0492 mL | 0.246 mL | 0.492 mL | 0.984 mL | 1.23 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.246 mL | 0.492 mL | 0.615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ARP 101 is a selective MMP-2 inhibitor with IC50 value of 0.81nM.
MMP-2 is an enzyme and involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction and tissue remodeling, as well as in disease processes, such as arthritis and metastasis [1].
ARP 101 shows inhibition of fibrosarcoma HT1080 cell growth in vitro. ARP 101 has shown ~250 fold selectivity of MMP-2 over MMP-1 and ~100 fold of MMP-9 over MMP-1 [2]. ARP 101 proved to be 15.3 and 24.7 times more potent than compound a and CGS 27023A on MMP-2, maintaining a different selectivity and potency of MMP-2/MMP-1 compared with inhibitor Prinomastat and CGS 27023A [3].
Reference:
[1]. Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol, 2007, 8(3): 221-33.
[2]. Rossello A, Nuti E, Orlandini E, et al. New N-arylsulfonyl-N-alkoxyaminoacetohydroxamic acids as selective inhibitors of gelatinase A (MMP-2). Bioorg Med Chem, 2004, 12(9): 2441-50.
[3]. Tuccinardi T, Martinelli A, Nuti E, et al. Amber force field implementation, molecular modelling study, synthesis and MMP-1/MMP-2 inhibition profile of (R)- and (S)-N-hydroxy-2-(N-isopropoxybiphenyl-4-ylsulfonamido)-3 methylbutanamides. Bioorg Med Chem, 2004, 14: 4260-4276.
- Deapi-platycoside E
Catalog No.:BCN3320
CAS No.:849758-42-5
- Rubicordifolin
Catalog No.:BCN7154
CAS No.:849699-55-4
- Y 134
Catalog No.:BCC7451
CAS No.:849662-80-2
- SU14813 maleate
Catalog No.:BCC1973
CAS No.:849643-15-8
- Blepharotriol
Catalog No.:BCN8056
CAS No.:849590-42-7
- Cefquinome
Catalog No.:BCC8910
CAS No.:84957-30-2
- Cevipabulin
Catalog No.:BCC6449
CAS No.:849550-05-6
- Cudraxanthone B
Catalog No.:BCN4398
CAS No.:84955-05-5
- Rosarin
Catalog No.:BCN5967
CAS No.:84954-93-8
- Rosavin
Catalog No.:BCN5968
CAS No.:84954-92-7
- TMPH hydrochloride
Catalog No.:BCC7360
CAS No.:849461-91-2
- 1-(4-Fluorobenzyl)-2-chlorobenzimidazole
Catalog No.:BCC8407
CAS No.:84946-20-3
- Maohuoside B
Catalog No.:BCN8084
CAS No.:849834-04-4
- 2-Benzoylbenzoic acid
Catalog No.:BCC8561
CAS No.:85-52-9
- Sudan IV;Solvent Red 24
Catalog No.:BCN8379
CAS No.:85-83-6
- Sudan III
Catalog No.:BCN6962
CAS No.:85-86-9
- Altrenogest
Catalog No.:BCC4479
CAS No.:850-52-2
- Methyl 1,6-dihydroxy-3-methylxanthone-8-carboxylate
Catalog No.:BCN7473
CAS No.:85003-85-6
- 5-Methoxysuberenone
Catalog No.:BCN3638
CAS No.:85011-58-1
- Sulfatinib
Catalog No.:BCC8811
CAS No.:1308672-74-3
- Afatinib dimaleate
Catalog No.:BCC1330
CAS No.:850140-73-7
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
- Shikonofuran A
Catalog No.:BCN2826
CAS No.:85022-66-8
- Cinchonain IIb
Catalog No.:BCN7738
CAS No.:85022-68-0
Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration.[Pubmed:28756707]
Expert Opin Emerg Drugs. 2017 Sep;22(3):235-246.
INTRODUCTION: Evolving anti-vascular endothelial growth factor (VEGF) treatments for neovascular age-related macular degeneration (nAMD) include long acting agents, combination strategies involving new pathways, topical agents, sustained-release, and genetic therapy strategies. Areas covered: Brolucizumab and abicipar pegol have smaller molecular size, facilitating higher concentrations and potentially longer duration than current anti-VEGF agents. Agents being combined with anti-VEGFs include OPT-302 (to inhibit VEGF-C and VEGF-D); pegpleranib and rinucumab (to inhibit platelet derived growth factor, PDGF - but both failed to show consistently improved visual outcomes compared to anti-VEGF monotherapy); and RG7716, ARP-1536 and nesvacumab (to activate the Tie-2 tyrosine kinase receptor, which reduces permeability). X-82 is an oral anti-VEGF and anti-PDGF being tested in phase 2 studies. Topical anti-VEGF +/- anti-PDGF drugs under study include pazopanib, PAN-90806, squalamine lactate, regorafinib, and LHA510. Sustained-release anti-VEGF delivery treatments, such as the ranibizumab Port Delivery System, GB-102, NT-503, hydrogel depot, Durasert, and ENV1305 aim to reduce the burden of frequent injections. Gene therapies with new viral vectors hold the potential to induce sustained expression of anti-angiogenic proteins via the retina's cellular apparatus, and include AVA-101/201, ADVM-202/302, AAV2-sFLT01, RGX314, and Retinostat. Expert opinion: There are many emerging anti-VEGF treatments that aim to improve visual outcomes and reduce the treatment burden of nAMD.
miR-155 promotes cutaneous wound healing through enhanced keratinocytes migration by MMP-2.[Pubmed:28247149]
J Mol Histol. 2017 Apr;48(2):147-155.
Inflammation, re-epithelization and tissue remodeling are three essential steps during wound healing. The re-epithelization process plays the most important role which mainly involves keratinocyte proliferation and migration. miR-155 has been reported to participate in cell migration and transformation, however, its function in skin wound healing is largely unknown. Here we hypothesize that overexpression of miR-155 at wound edges could accelerate wound healing mediated by enhanced keratinocyte migration. To test this hypothesis, direct local injection of miR-155 expression plasmid to wound edges was conducted to overexpress miR-155 in vivo. Results shown that miR-155 significantly promoted wound healing and re-epithelization compared to control, while did not affect wound contraction. Also, miR-155 overexpression accelerated primarily cultured keratinocyte migration in vitro, but had no effect on cell proliferation. Importantly, western blot analysis shown that MMP-2 was significantly upregulated whiles its inhibitor TIMP-1 downregulated after miR-155 treatment. Moreover, the use of ARP-101, an MMP-2 inhibitor, effectively attenuated the accelerative effects on cell migration induced by miR-155. Taken together, our results suggest that miR-155 has the promote effect on wound healing that is probably mediated by accelerating keratinocyte migration via upregulated MMP-2 level. This study provides a rationale for the therapeutic effect of miR-155 on wound healing.
Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture.[Pubmed:26367794]
Nature. 2015 Oct 1;526(7571):112-7.
The extent to which low-frequency (minor allele frequency (MAF) between 1-5%) and rare (MAF
Phenotypic characterization of the CRISPA (ARP gene) mutant of pea (Pisum sativum; Fabaceae): a reevaluation.[Pubmed:24638162]
Am J Bot. 2014 Mar;101(3):408-27.
PREMISE OF THE STUDY: Leaf form and development are controlled genetically. The ARP genes encode MYB transcription factors that interact with Class 1 KNOX genes in a regulatory module that controls meristem-leaf determinations and is highly conserved in plants. ARP loss of function alleles and subsequent KNOX1 overexpression cause many unusual leaf phenotypes including loss or partial loss of the ability to produce a lamina and production of "knots" on leaf blades. CRISPA (CRI) is the ARP gene in pea, and a number of its mutant alleles are known. METHODS: We made morphological and anatomical evaluations of cri-1 mutant plants while controlling for genetic background and for heteroblastic effects, and we used aldehyde fixation and resin preparations for anatomical analysis. Further, we compared gene expression in WT and cri-1 shoot tips and HOP1/PsKN1 and CRI expression in other leaf mutants. KEY RESULTS: The cri-1 plants had more extensive abnormalities in the proximal than in the distal regions of the leaf, including ectopic stipules, narrow leaflets, and shortened petioles with excessive adaxial expansion. "Knots" were morphologically and anatomically variable but consisted of vascularized out-pocketing of the adaxial leaflet surface. HOP1/PsKN1 and UNI mRNA levels were higher in cri-1 shoot tips, and some auxin-regulated genes were lower. Low LE expression suggests that the GA level is high in cri-1 shoot tips. CONCLUSIONS: The CRISPA gene of pea suppresses KNOX1 genes and UNI and functions to (1) maintain proximal-distal regions in their appropriate positions, (2) restrict excessive adaxial cell proliferation, and (3) promote laminar expansion.
Roles of pro-angiogenic and anti-angiogenic factors as well as matrix metalloproteinases in healing of NSAID-induced small intestinal ulcers in rats.[Pubmed:23900029]
Life Sci. 2013 Oct 6;93(12-14):441-7.
AIMS: We examined changes in the expression of a pro-angiogenic factor, vascular endothelial growth factor (VEGF), and an anti-angiogenic factor, endostatin, as well as matrix metalloproteinase (MMP)-2 and MMP-9 in the rat small intestine after administration of indomethacin and investigated the roles of these factors in the healing of indomethacin-induced small intestinal ulcers. MAIN METHODS: Male SD rats were given indomethacin (10mg/kg) p.o. and euthanized at various time points (3-24h and 2-7days) after the administration. To impair the healing of these lesions, low-dose of indomethacin (2mg/kg) was given p.o. once daily for 6days starting 1day after ulceration. Levels of VEGF, endostatin, MMP-2 and MMP-9 were determined by Western blotting. KEY FINDINGS: The expression of both VEGF and endostatin was upregulated after the ulceration. Repeated administration of low-dose indomethacin impaired the ulcer healing with a decrease of VEGF expression and a further increase of endostatin expression, resulting in a marked decrease in the ratio of VEGF/endostatin expression. The levels of MMP-2 and MMP-9 were both significantly increased after the ulceration, but these responses were suppressed by the repeated indomethacin treatment. The healing of these ulcers was significantly delayed by the repeated administration of MMP inhibitors such as ARP-101 and SB-3CT. SIGNIFICANCE: The results confirm the importance of the balance between pro-angiogenic and anti-angiogenic activities in the healing of indomethacin-induced small intestinal damage and further suggest that the increased expression of MMP-2 and MMP-9 is another important factor for ulcer healing in the small intestine.
Amber force field implementation, molecular modelling study, synthesis and MMP-1/MMP-2 inhibition profile of (R)- and (S)-N-hydroxy-2-(N-isopropoxybiphenyl-4-ylsulfonamido)-3-methylbutanamides.[Pubmed:16483784]
Bioorg Med Chem. 2006 Jun 15;14(12):4260-76.
Ab initio calculations (B3LYP/Lanl2DZ level of theory) were performed in this study to determine all the structural and catalytic zinc parameters required in order to study MMPs and their complexes with hydroxamate inhibitors by means of the AMBER force field. The parameters thus obtained were used in order to study the docking of some known MMPi (Batimastat, CGS 27023A and Prinomastat) and our previously described inhibitor a which had shown an inhibitory activity for MMP-1, and -2, with the aim of explaining the different selectivity. On this basis the two enantiomers (R)-b and (S)-b were designed and synthesized, as more potent MMP-2 inhibitors than our previously described inhibitor a. Between these two enantiomers the eutomer (R)-b proved to be 24.7 times and 15.3 times more potent than CGS 27023A and the parent compound a on MMP-2, maintaining a higher index of MMP-2/MMP-1 selectivity compared with CGS 27023A and the more potent inhibitor Prinomastat. The hydroxamate (R)-b can be considered as a progenitor of a new class of biphenylsulfonamido-based inhibitors that differ from compound a in the presence of an alkyl side chain on the C alpha atom, and show different potency and selectivity profiles on the two MMPs considered.


