Ro 32-3555MMP inhibitor CAS# 190648-49-8 |
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 190648-49-8 | SDF | Download SDF |
| PubChem ID | 193987 | Appearance | Powder |
| Formula | C22H35N4O5 | M.Wt | 435.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Cipemastat | ||
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
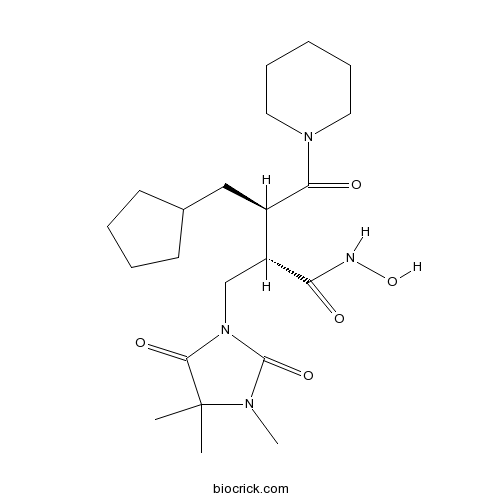
| Chemical Name | (2S,3R)-3-(cyclopentylmethyl)-N-hydroxy-4-oxo-4-piperidin-1-yl-2-[(3,4,4-trimethyl-2,5-dioxoimidazolidin-1-yl)methyl]butanamide | ||
| SMILES | CC1(C(=O)N(C(=O)N1C)CC(C(CC2CCCC2)C(=O)N3CCCCC3)C(=O)NO)C | ||
| Standard InChIKey | GFUITADOEPNRML-IAGOWNOFSA-N | ||
| Standard InChI | InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cipemastat is a potent, competitive inhibitor of human collagenases 1, 2 and 3 with Kis of 3.0, 4.4 and 3.4 nM, respectively.Cipemastat (Ro 32-3555) is a potent, competitive inhibitor of human matrix metalloproteinases. Cipemastat is selective for collagenase 1, 2 and 3 relative to related matrix metalloproteinases. Cipemastat is also a potent inhibitor of rat collagenase (IC50=44.7±3.4 nM (n=4)). In vitro cartilage degradation ± inhibited IL-1a induced cartilage degradation in vitro in a concentration-dependent manner with an IC50=60 nM. The inhibition is not mediated by a cytotoxic action on explant chondrocytes. Cipemastat, at all concentrations tested, fail to modify glucose utilization when compared to explants cultured in the presence of IL-La alone.The amount of hydroxyproline in non-implanted cartilage is 119.3±4.2 nM/mg and this decreases in cartilages implanted in vehicle-dosed animals to 53.6±7.1 nM/mg over a fourteen day period. Animals administered Cipemastat orally at doses of 2.5, 5, 10 and 25 mg/kg show statistically increased levels of implanted cartilage hydroxypro-line. Fourteen days after the second challenge injection of P. acnes, the area of cartilage most consistently affected by pannus is the lateral femoral condyle, which is the area analysed. In non-arthritic animals the mean cartilage area is 0.17±0.02 mm2 (n=5). In arthritic animals there is a significant decrease to a mean area of 0.086±0.01 mm2 (n=10). The group of animals dosed with Cipemastat (50 mg/kg, p.o.) show a significantly greater area of cartilage with a mean value of 0.126±0.012 mm2 (n=9). The pannus area in vehicle-dosed animals is 0.099±0.017 mm2 and in Cipemastat dosed animals 0.102±0.019 mm2. Adjuvant arthritis injection of adjuvant induced two phases of swelling of the injected paw in vehicle-dosed rats. The primary swelling phase occurred between days 0 to 5 and induced an increase in paw volume of 1.9±0.1 mL; the secondary phase occurrs between day 9 to 14 and there was an increase in paw swelling of 0.98±0.08 mL. The group of animals dosed with dexamethasone (0.1 mg/kg) shows a significant reduction in both primary (0.2±0.03 mL) and secondary inflammation (0.07±0.08 mL) paw swelling as well as total inhibition of the lesion score |

Ro 32-3555 Dilution Calculator

Ro 32-3555 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2962 mL | 11.4811 mL | 22.9621 mL | 45.9242 mL | 57.4053 mL |
| 5 mM | 0.4592 mL | 2.2962 mL | 4.5924 mL | 9.1848 mL | 11.4811 mL |
| 10 mM | 0.2296 mL | 1.1481 mL | 2.2962 mL | 4.5924 mL | 5.7405 mL |
| 50 mM | 0.0459 mL | 0.2296 mL | 0.4592 mL | 0.9185 mL | 1.1481 mL |
| 100 mM | 0.023 mL | 0.1148 mL | 0.2296 mL | 0.4592 mL | 0.5741 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Potent, collagenase-selective MMP inhibitor (Ki values are 3.0, 3.4, 4.4, 59, 154 and 527 nM for MMP-1, MMP-13, MMP-8, MMP-9, MMP-2 and MMP-3 respectively). Inhibits cartilage breakdown in vitro and in vivo and displays antiarthritic activity. Orally active.
- Ac-Gly-OEt
Catalog No.:BCC2944
CAS No.:1906-82-7
- Prosapogenin A
Catalog No.:BCN2582
CAS No.:19057-67-1
- Dioscin
Catalog No.:BCN6273
CAS No.:19057-60-4
- AT 1015
Catalog No.:BCC6194
CAS No.:190508-50-0
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- 17β-Benzoyloxy-androsta-1,4-dien-3-one
Catalog No.:BCC8443
CAS No.:19041-66-8
- SLIGKV-NH2
Catalog No.:BCC3959
CAS No.:190383-13-2
- Orientanol A
Catalog No.:BCN4064
CAS No.:190381-82-9
- 3-Butyryloxytropane
Catalog No.:BCN1924
CAS No.:19038-34-7
- Sarracine N-oxide
Catalog No.:BCN2022
CAS No.:19038-27-8
- 7-Hydroxy-4-Methyl-8-Nitrocoumarin
Catalog No.:BCC9210
CAS No.:19037-69-5
- GR 135531
Catalog No.:BCC6836
CAS No.:190277-13-5
- Bepotastine Besilate
Catalog No.:BCC4538
CAS No.:190786-44-8
- Gracillin
Catalog No.:BCN5360
CAS No.:19083-00-2
- Isocupressic acid
Catalog No.:BCN1177
CAS No.:1909-91-7
- Triptocallic acid A
Catalog No.:BCN1176
CAS No.:190906-61-7
- Calystegine C2
Catalog No.:BCN1878
CAS No.:190957-44-9
- Benzyl 4-Oxo-1-piperidinecarboxylate
Catalog No.:BCC8870
CAS No.:19099-93-5
- Salvigenin
Catalog No.:BCN1178
CAS No.:19103-54-9
- L-168,049
Catalog No.:BCC7325
CAS No.:191034-25-0
- K-7174
Catalog No.:BCC6435
CAS No.:191089-60-8
- Kuguacin R
Catalog No.:BCN3057
CAS No.:191097-54-8
- Oplopanone
Catalog No.:BCN1179
CAS No.:1911-78-0
- Telithromycin
Catalog No.:BCC5273
CAS No.:191114-48-4
Alkylation of succinates: synthesis of Ro 32-3555.[Pubmed:9871642]
Bioorg Med Chem Lett. 1998 Jan 20;8(2):143-6.
A short synthesis, based on a succinate acylation-alkylation-decarboxylation approach, of the clinical compound Ro 32-3555 is reported. The nature of the selectivity in the mono-alkylation of succinates was examined under varying enolization conditions and in the presence of chaotropic additives.
Ro 32-3555, an orally active collagenase selective inhibitor, prevents structural damage in the STR/ORT mouse model of osteoarthritis.[Pubmed:9751097]
Arthritis Rheum. 1998 Sep;41(9):1639-44.
OBJECTIVE: To determine the efficacy of a selective inhibitor of collagenases in an animal model of osteoarthritis (OA). METHODS: Ro 32-3555, an orally active collagenase selective inhibitor, was administered to STR/ORT mice. Microfocal x-ray-generated images of the hind limbs were visually scored for joint space narrowing, osteophyte formation, and calcification of tendons. Histologic sections of the knees were scored for cartilage changes including loss of surface matrix, fibrillation, and eburnation. RESULTS: Significant inhibition of joint space narrowing and osteophyte formation was achieved in groups of animals treated with 10-50 mg/kg(-1) of Ro 32-3555. These effects were confirmed histologically in the same groups of animals: histologic analysis revealed that Ro 32-3555 protected cartilage from degradative changes. CONCLUSION: Ro 32-3555, a collagenase selective inhibitor, inhibits both the cartilage and bone changes in this mouse model of OA, and thus shows great potential as a treatment of OA in humans.
Ro 32-3555, an orally active collagenase inhibitor, prevents cartilage breakdown in vitro and in vivo.[Pubmed:9179398]
Br J Pharmacol. 1997 Jun;121(3):540-6.
1. Ro 32-3555 (3(R)-(cyclopentylmethyl)-2(R)-[(3,4,4-trimethyl-2,5-dioxo-1- imidazolidinyl)methyl]-4-oxo-4-piperidinobutyrohydroxamic acid) is a potent, competitive inhibitor of human collagenases 1, 2 and 3 (Ki values of 3.0, 4.4 and 3.4 nM, respectively). The compound is a selective inhibitor of collagenases over the related human matrix metalloproteinases stromelysin 1, and gelatinases A and B (Ki values of 527, 154 and 59 nM, respectively). 2. Ro 32-3555 inhibited interleukin-1 alpha (IL-1 alpha)-induced cartilage collagen degradation in vitro in bovine nasal cartilage explants (IC50 = 60 nM). 3. Ro 32-3555 was well absorbed in rats when administered orally. Systemic exposure was dose related, with an oral bioavailability of 26% at a dose of 25 mg kg-1. 4. Ro 32-3555 prevented granuloma-induced degradation of bovine nasal cartilage cylinders implanted subcutaneously into rats (ED50 = 10 mg kg-1, twice daily, p.o.). 5. Ro 32-3555 dosed once daily for 14 days at 50 mg kg-1, p.o., inhibited degradation of articular cartilage in a rat monoarthritis model induced by an intra-articular injection of Propionibacterium acnes. 6. Ro 32-3555 is a potential therapy for the treatment of the chronic destruction of articulating cartilage in both rheumatoid and osteoarthritis.
Cartilage protective agent (CPA) Ro 32-3555, a new matrix metalloproteinase inhibitor for the treatment of rheumatoid arthritis.[Pubmed:9426828]
Agents Actions Suppl. 1998;49:49-55.
CPA was well tolerated at all dose levels (10-150 mg) following single oral dose administration to healthy male volunteers. There was no relationship between the intensity, duration and number of adverse events reported and the dose of CPA. There was a dose-related increase in exposure as measured by AUC0-infinity and Cmax. Administration of 10 mg CPA following food resulted in a delayed tmax, and a significant decrease in Cmax but not AUC0-infinity.


