Bepotastine BesilateCAS# 190786-44-8 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 190786-44-8 | SDF | Download SDF |
| PubChem ID | 444016 | Appearance | Powder |
| Formula | C27H31ClN2O6S | M.Wt | 547.06 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (182.80 mM) *"≥" means soluble, but saturation unknown. | ||
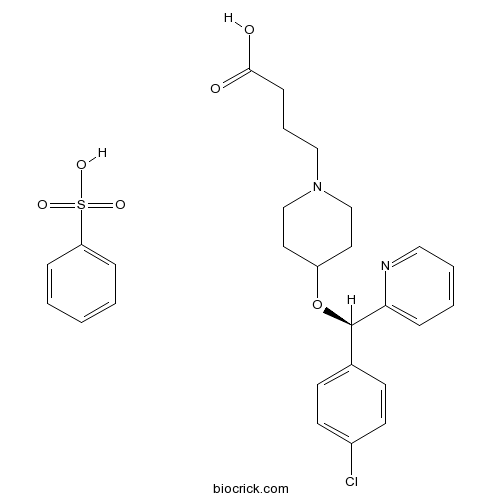
| Chemical Name | benzenesulfonic acid;4-[4-[(R)-(4-chlorophenyl)-pyridin-2-ylmethoxy]piperidin-1-yl]butanoic acid | ||
| SMILES | C1CN(CCC1OC(C2=CC=C(C=C2)Cl)C3=CC=CC=N3)CCCC(=O)O.C1=CC=C(C=C1)S(=O)(=O)O | ||
| Standard InChIKey | UDGHXQPQKQPSBB-ZMBIFBSDSA-N | ||
| Standard InChI | InChI=1S/C21H25ClN2O3.C6H6O3S/c22-17-8-6-16(7-9-17)21(19-4-1-2-12-23-19)27-18-10-14-24(15-11-18)13-3-5-20(25)26;7-10(8,9)6-4-2-1-3-5-6/h1-2,4,6-9,12,18,21H,3,5,10-11,13-15H2,(H,25,26);1-5H,(H,7,8,9)/t21-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bepotastine Beslilate (Bepreve) is a histamine H1 receptor anatagonist.
IC50 value:
Target: Histamine H1 receptor
Bepotastine Beslilate (Bepreve) also suppresses some allergic inflammatory processes such as allergic rhinitis, chronic urticaria or pruritus associated with skin conditions (eczema/dermatitis, prurigo or pruritus cutaneus).Bepotastine Beslilate (Bepreve) is useful for allergic conjunctivitis. References: | |||||

Bepotastine Besilate Dilution Calculator

Bepotastine Besilate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.828 mL | 9.1398 mL | 18.2795 mL | 36.5591 mL | 45.6988 mL |
| 5 mM | 0.3656 mL | 1.828 mL | 3.6559 mL | 7.3118 mL | 9.1398 mL |
| 10 mM | 0.1828 mL | 0.914 mL | 1.828 mL | 3.6559 mL | 4.5699 mL |
| 50 mM | 0.0366 mL | 0.1828 mL | 0.3656 mL | 0.7312 mL | 0.914 mL |
| 100 mM | 0.0183 mL | 0.0914 mL | 0.1828 mL | 0.3656 mL | 0.457 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bepotastine Beslilate (Bepreve) is a histamine H1 receptor anatagonist.
- Ro 32-3555
Catalog No.:BCC2377
CAS No.:190648-49-8
- Ac-Gly-OEt
Catalog No.:BCC2944
CAS No.:1906-82-7
- Prosapogenin A
Catalog No.:BCN2582
CAS No.:19057-67-1
- Dioscin
Catalog No.:BCN6273
CAS No.:19057-60-4
- AT 1015
Catalog No.:BCC6194
CAS No.:190508-50-0
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- 17β-Benzoyloxy-androsta-1,4-dien-3-one
Catalog No.:BCC8443
CAS No.:19041-66-8
- SLIGKV-NH2
Catalog No.:BCC3959
CAS No.:190383-13-2
- Orientanol A
Catalog No.:BCN4064
CAS No.:190381-82-9
- 3-Butyryloxytropane
Catalog No.:BCN1924
CAS No.:19038-34-7
- Sarracine N-oxide
Catalog No.:BCN2022
CAS No.:19038-27-8
- 7-Hydroxy-4-Methyl-8-Nitrocoumarin
Catalog No.:BCC9210
CAS No.:19037-69-5
- Gracillin
Catalog No.:BCN5360
CAS No.:19083-00-2
- Isocupressic acid
Catalog No.:BCN1177
CAS No.:1909-91-7
- Triptocallic acid A
Catalog No.:BCN1176
CAS No.:190906-61-7
- Calystegine C2
Catalog No.:BCN1878
CAS No.:190957-44-9
- Benzyl 4-Oxo-1-piperidinecarboxylate
Catalog No.:BCC8870
CAS No.:19099-93-5
- Salvigenin
Catalog No.:BCN1178
CAS No.:19103-54-9
- L-168,049
Catalog No.:BCC7325
CAS No.:191034-25-0
- K-7174
Catalog No.:BCC6435
CAS No.:191089-60-8
- Kuguacin R
Catalog No.:BCN3057
CAS No.:191097-54-8
- Oplopanone
Catalog No.:BCN1179
CAS No.:1911-78-0
- Telithromycin
Catalog No.:BCC5273
CAS No.:191114-48-4
- Atrazine
Catalog No.:BCC8838
CAS No.:1912-24-9
Double-blind placebo-controlled study of bepotastine besilate in pediatric patients with perennial allergic rhinitis.[Pubmed:26364765]
Expert Opin Pharmacother. 2015;16(16):2395-408.
BACKGROUND: Although second-generation antihistamines, such as Bepotastine Besilate, are recommended as a first-line treatment option for adult perennial allergic rhinitis (PAR), few non-sedating second-generation antihistamines are safe for children. OBJECTIVE: A double-blind, placebo-controlled, comparative study of 473 pediatric PAR patients (7 - 15 years old) to determine the superiority and safety of Bepotastine Besilate (10 mg twice daily) relative to placebo for improved total and individual nasal symptom scores compared with baseline. RESEARCH DESIGN AND METHODS: Subjects were randomized to placebo (n = 233) or Bepotastine Besilate (n = 240, 10 mg orally twice daily for 2 weeks). Interference of daily life by PAR was assessed by measuring change in individual nasal symptom scores from baseline. RESULTS: Bepotastine Besilate was superior to placebo in terms of total nasal symptom scores, with improved overall nasal symptoms of PAR compared with baseline values. Subgroup analyses demonstrated Bepotastine Besilate was effective irrespective of age, sex or body weight. No clinically significant adverse drug reactions often observed with first-generation antihistamines were reported and no difference in adverse events between groups was observed. CONCLUSIONS: Bepotastine Besilate is effective and safe for pediatric PAR patients aged 7 - 15 years, and has a significant clinical impact on PAR. CLINICAL TRIAL REGISTRATION: ClinicalTrials.gov identifier: NCT01861522 ( https://clinicaltrials.gov/ct2/show/NCT01861522 ).
Treatment of allergic conjunctivitis with bepotastine besilate ophthalmic solution 1.5%.[Pubmed:25152611]
Clin Ophthalmol. 2014 Aug 13;8:1495-505.
PURPOSE: To examine the pooled per-protocol ocular end points from two conjunctival allergen challenge (CAC) clinical trials of the dual-action antihistamine Bepotastine Besilate ophthalmic solution (BBOS) 1.5%. METHODS: Two Phase III, placebo-controlled, double-masked, randomized clinical trials were conducted at a total of six separate centers using the CAC model of allergic conjunctivitis. The same study design was employed for both clinical trials, with subjects randomly assigned to either BBOS 1.5% (n=78) or placebo (n=79) treatment. Each subject received one eye drop of the test agent bilaterally at different study visits 15 minutes, 8 hours, or 16 hours prior to a CAC. Primary ocular end points included changes in ocular itching reported at 3, 5, and 7 minutes and conjunctival hyperemia assessed at 7, 15, and 20 minutes following each CAC. Secondary ocular end points included chemosis as well as episcleral and ciliary hyperemia judged by investigators, and tearing (scored as either absent or present) and eyelid swelling judged by subjects. RESULTS: A statistically significant reduction in ocular itching was observed for BBOS 1.5% treatment compared to placebo at all time points (P<0.0001), while measures for onset and 8-hour persistence of action also reached clinical significance (ie, >/=1.0 unit difference) at a majority of time points. In addition, a significant reduction in conjunctival hyperemia was achieved at a majority of time points during the onset of action CAC test. Secondary end points were also significantly improved compared to placebo, most prominently for reduced tearing at all study visits and reduced eyelid swelling at the onset of action and 8-hour study visits. Adverse events were generally mild and transient. CONCLUSION: BBOS 1.5% rapidly reduced CAC-induced ocular itching with duration of effectiveness of at least 8 hours after dosing. Certain secondary signs of inflammation were also significantly reduced.
Bepotastine besilate for the treatment of pruritus.[Pubmed:24191914]
Expert Opin Pharmacother. 2013 Dec;14(18):2553-69.
INTRODUCTION: Bepotastine Besilate 1.5% is a newly approved second-generation topical antihistamine indicated for the pruritus associated with allergic conjunctivitis. In Japan, the oral formulation is approved to manage pruritus associated with allergic rhinitis and urticaria. AREAS COVERED: Bepotastine is a piperidine derivative that antagonizes H1 receptors with high selectivity. It has been labeled a dual-acting or multiple-acting antiallergic medication, because it inhibits histamine at H1 receptors and stabilizes mast cells to prevent histamine release. Bepotastine may also have other immunoactive properties, such as inhibition of eosinophil migration, interleukin-5 (IL-5), leukotrienes (e.g., LTB4) and platelet-activating factor (PAF). Human clinical trials demonstrate the efficacy and safety of systemic and ophthalmic bepotastine for pruritus relief, limited penetration across the blood-brain-barrier and kinetics suitable for twice-daily administration. EXPERT OPINION: Bepotastine Besilate 1.5% ophthalmic solution is a safe and effective treatment option for allergic conjunctivitis associated pruritus. Side-effect profile is similar to other ocular antihistamine agents. Additional comparative-effectiveness studies would further advance its clinical use. Oral bepotastine is a safe and effective treatment option approved in Japan for allergic rhinitis, urticaria and pruritus associated with skin diseases.
Pharmacokinetic comparisons of bepotastine besilate and bepotastine salicylate in healthy subjects.[Pubmed:24105252]
Clin Drug Investig. 2013 Dec;33(12):913-9.
BACKGROUND AND OBJECTIVE: Bepotastine is a second-generation histamine H(1) receptor antagonist that is indicated in allergic rhinitis, urticaria, and pruritus associated with skin disease. The aim of the present study was to compare the pharmacokinetics of two different bepotastine formulations [Bepotastine Besilate 10 mg (reference) and bepotastine salicylate 9.64 mg (test)], both containing 7.11 mg bepotastine base, to satisfy regulatory requirements for marketing. METHODS: A single-center, randomized, single-dose, open-label, two-period, two-sequence crossover study with a 7-day washout period was conducted in 26 healthy male subjects. Plasma samples for drug analysis were collected up to 24 h after drug treatment. Pharmacokinetic parameters, including maximum plasma concentration (C(max)) and area under the plasma concentration-time curve (AUC), were calculated. ANOVA for bioequivalence was conducted using log-transformed C(max) and AUC values, and the mean ratios and their 90 % confidence intervals were calculated. RESULTS: Of the 26 participants initially enrolled, 24 healthy participants completed both treatment periods. All pharmacokinetic parameters of bepotastine exhibited no significant differences between the two formulations. The observed mean (standard deviation) C(max), AUC from time zero to the time of the last measurable concentration (AUC(last)), and AUC from time zero to infinity (AUC(infinity)) values for the reference formulation were 99.9 (31.4) ng/mL, 388.9 (102.6) ng.h/mL, and 392.4 (103.6) ng.h/mL, respectively. Corresponding values for the test formulation were 101.0 (26.3) ng/mL, 389.8 (112.2) ng.h/mL, and 393.7 (111.7) ng.h/mL. The geometric mean ratios (90 % CI) between the two formulations were 1.0220 (0.9224-1.1324) for C(max), 0.9928 (0.9521-1.0351) for AUC(last), and 0.9959 (0.9549-1.0387) for AUC(infinity). During the study period, two adverse events were reported in the test formulation group, but both were transient, mild, and resolved completely during the treatment period. These adverse events were considered unrelated to the study drugs. CONCLUSION: The results of the present study revealed that Bepotastine Besilate 10 mg (reference) and bepotastine salicylate 9.64 mg (test) formulations have comparable pharmacokinetic characteristics and that these two formulations meet the regulatory criteria for bioequivalence. Both bepotastine formulations were generally well-tolerated in this population.


