AT 1015Long-acting 5-HT2A antagonist CAS# 190508-50-0 |
- Sabutoclax
Catalog No.:BCC2236
CAS No.:1228108-65-3
- ABT-199
Catalog No.:BCC3614
CAS No.:1257044-40-8
- WEHI-539
Catalog No.:BCC2055
CAS No.:1431866-33-9
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- ABT-737
Catalog No.:BCC3613
CAS No.:852808-04-9
- TW-37
Catalog No.:BCC2257
CAS No.:877877-35-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 190508-50-0 | SDF | Download SDF |
| PubChem ID | 9805663 | Appearance | Powder |
| Formula | C29H34ClN3O2 | M.Wt | 492.05 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
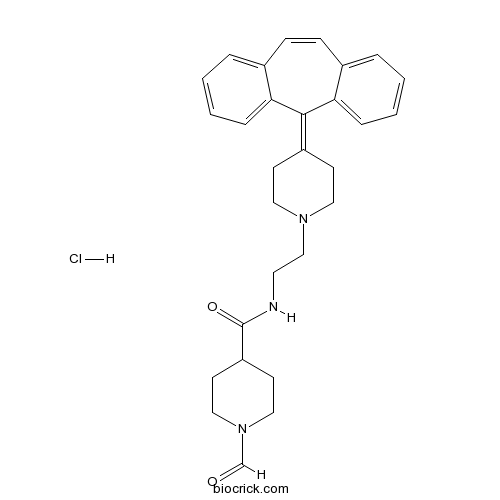
| Chemical Name | N-[2-[4-(dibenzo[1,2-a:1',2'-e][7]annulen-11-ylidene)piperidin-1-yl]ethyl]-1-formylpiperidine-4-carboxamide;hydrochloride | ||
| SMILES | C1CN(CCC1C(=O)NCCN2CCC(=C3C4=CC=CC=C4C=CC5=CC=CC=C53)CC2)C=O.Cl | ||
| Standard InChIKey | YZHGXYNLMHGNJZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H33N3O2.ClH/c33-21-32-18-13-25(14-19-32)29(34)30-15-20-31-16-11-24(12-17-31)28-26-7-3-1-5-22(26)9-10-23-6-2-4-8-27(23)28;/h1-10,21,25H,11-20H2,(H,30,34);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Long-acting 5-HT2A receptor antagonist; inhibits vasoconstriction and blocks platelet aggregation. Prolongs clotting time in a rat model of thrombus formation; ameliorates laurate-induced peripheral vascular lesions in rodents. |

AT 1015 Dilution Calculator

AT 1015 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0323 mL | 10.1616 mL | 20.3231 mL | 40.6463 mL | 50.8078 mL |
| 5 mM | 0.4065 mL | 2.0323 mL | 4.0646 mL | 8.1293 mL | 10.1616 mL |
| 10 mM | 0.2032 mL | 1.0162 mL | 2.0323 mL | 4.0646 mL | 5.0808 mL |
| 50 mM | 0.0406 mL | 0.2032 mL | 0.4065 mL | 0.8129 mL | 1.0162 mL |
| 100 mM | 0.0203 mL | 0.1016 mL | 0.2032 mL | 0.4065 mL | 0.5081 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- 17β-Benzoyloxy-androsta-1,4-dien-3-one
Catalog No.:BCC8443
CAS No.:19041-66-8
- SLIGKV-NH2
Catalog No.:BCC3959
CAS No.:190383-13-2
- Orientanol A
Catalog No.:BCN4064
CAS No.:190381-82-9
- 3-Butyryloxytropane
Catalog No.:BCN1924
CAS No.:19038-34-7
- Sarracine N-oxide
Catalog No.:BCN2022
CAS No.:19038-27-8
- 7-Hydroxy-4-Methyl-8-Nitrocoumarin
Catalog No.:BCC9210
CAS No.:19037-69-5
- GR 135531
Catalog No.:BCC6836
CAS No.:190277-13-5
- Bripiodionene
Catalog No.:BCN1831
CAS No.:190265-70-4
- Spiratisanin C
Catalog No.:BCN6975
CAS No.:1902173-22-1
- Spiratisanin B
Catalog No.:BCN6976
CAS No.:1902173-19-6
- Spiratisanin A
Catalog No.:BCN6977
CAS No.:1902173-16-3
- Dioscin
Catalog No.:BCN6273
CAS No.:19057-60-4
- Prosapogenin A
Catalog No.:BCN2582
CAS No.:19057-67-1
- Ac-Gly-OEt
Catalog No.:BCC2944
CAS No.:1906-82-7
- Ro 32-3555
Catalog No.:BCC2377
CAS No.:190648-49-8
- Bepotastine Besilate
Catalog No.:BCC4538
CAS No.:190786-44-8
- Gracillin
Catalog No.:BCN5360
CAS No.:19083-00-2
- Isocupressic acid
Catalog No.:BCN1177
CAS No.:1909-91-7
- Triptocallic acid A
Catalog No.:BCN1176
CAS No.:190906-61-7
- Calystegine C2
Catalog No.:BCN1878
CAS No.:190957-44-9
- Benzyl 4-Oxo-1-piperidinecarboxylate
Catalog No.:BCC8870
CAS No.:19099-93-5
- Salvigenin
Catalog No.:BCN1178
CAS No.:19103-54-9
- L-168,049
Catalog No.:BCC7325
CAS No.:191034-25-0
Survival and freedom from aortic valve-related reoperation after valve-sparing aortic root replacement in 1015 patients.[Pubmed:26718320]
Interact Cardiovasc Thorac Surg. 2016 Apr;22(4):431-8.
OBJECTIVES: The aim of this study was to characterize mortality and aortic valve replacement after valve-sparing aortic root replacement (V-SARR) in a multicentre cohort. METHODS: Between 1994 and 2014, 1015 patients had V-SARR with (n = 288, 28%) or without cusp/commissure repair (n = 727, 72%) at the centres of Lubeck (n = 343, 34%), Stuttgart (n = 346, 34%), Hamburg (n = 109, 11%) and Freiburg (n = 217, 21%), Germany. Comparative survival of an age- and gender-matched general population was calculated. Log-rank tests and multiple logistic regression were used to identify risk factors. RESULTS: The mean follow-up was 5.2 +/- 3.9 years. Cumulative follow-up comprised 2933 patient-years. Early survival was 98%. NYHA status and aneurysm size were predictive of death during mid-term follow-up (P = 0.025). Freedom from aortic valve replacement was 90% at 8 years, with the type of V-SARR (root remodelling, David II) being a risk factor (P = 0.015). Bicuspid aortic valve (P = 0.26) and initial valve function (P = 0.4) did not impact reoperation. The need of additional valve repair (cusps/commissures) was not linked to reoperation: freedom from aortic valve replacement at 8 years was 84% if cusp repair was performed versus 90% if V-SARR alone was performed (P = 0.218). Marfan syndrome had no impact on survival or on aortic valve replacement. CONCLUSIONS: Mid-term survival of patients after V-SARR is comparable with that of a matched general population. The regurgitant bicuspid aortic valve is a favourable substrate for V-SARR. Prophylactic surgery should be performed before symptoms or large aneurysms are present to achieve optimal mid-term outcomes.
Effector-driven marker development and cloning of resistance genes against Phytophthora infestans in potato breeding clone SW93-1015.[Pubmed:26518573]
Theor Appl Genet. 2016 Jan;129(1):105-15.
KEY MESSAGE: We show the usefulness of integrating effector screening in a breeding program and in resistance gene cloning, with Phytophthora resistance in the Swedish potato breeding clone SW93-1015 as an example. Phytophthora infestans is one of the most devastating plant pathogens worldwide. We have earlier found that the SW93-1015 potato breeding clone has an efficient resistance against P. infestans under field conditions in Sweden, which has an unusually high local diversity of the pathogen. This potato clone has characteristics that are different from classical R-gene-mediated resistance such as elevated levels of hydrogen peroxide (H2O2) under controlled conditions. Analysis of 76 F1 potato progenies from two individual crosses resulted in nearly 50% resistant clones, from both crosses. This result suggests that the SW93-1015 clone has a simplex genotype for this trait. Screening with over 50 different P. infestans effectors, containing the conserved motif RXLR (for Arg, any amino acid, Leu, Arg), revealed a specific response to Avr2, which suggests that SW93-1015 might contain a functional homolog of the R2 resistance gene. We cloned eight R2 gene homologs from SW93-1015, whereof seven have not been described before and one gene encoded a protein identical to Rpi-ABPT. Expression of this gene in potato cultivar Desiree provided R2-specific resistance, whereas other homologues did not. Using RNAseq analyses we designed a new DNA marker for the R2 resistance in SW93-1015. In summary, we have demonstrated the use of effector screening in practical breeding material and revealed the key resistance mechanism for SW93-1015.
Antithrombotic activity of AT-1015, a potent 5-HT(2A) receptor antagonist, in rat arterial thrombosis model and its effect on bleeding time.[Pubmed:11755147]
Eur J Pharmacol. 2001 Dec 21;433(2-3):157-62.
The antithrombotic activity of N-[2-(4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)piperidino)ethyl]-1-formyl-4-piperi dinecarboxamide monohydrochloride monohydrate (AT-1015; a 5-HT(2A) receptor antagonist) was studied in a photochemically induced arterial thrombosis (PIT) model in the rat femoral artery, and in the tail transection bleeding time test. Ticlopidine (an antiplatelet agent) and sarpogrelate (a selective 5-HT(2A) receptor antagonist) were studied as reference compounds. Pretreatment with AT-1015 (1 mg/kg, p.o.) significantly prolonged the time required to occlusion of the artery with thrombus, and the effect (3 mg/kg, p.o.) persisted for 24 h with significant inhibition of 5-HT-induced vascular contraction. Ticlopidine and sarpogrelate also significantly prolonged the time to occlusion at 100 mg/kg, p.o. Sarpogrelate (300 mg/kg, p.o.) showed the similar antithrombotic efficacy to AT-1015 (3 mg/kg, p.o.), while the effect disappeared within 6 h. No significant bleeding time prolongation was observed at 10 mg/kg of AT-1015, which is 10 times higher than the antithrombotic effective dose; whereas ticlopidine significantly prolonged bleeding time at the same dose as the antithrombotic effective dose. These results suggested that AT-1015 is a potent and long-acting oral antithrombotic agent in this model, which may be elucidated by its potent and long-acting inhibition of vasoconstriction through 5-HT(2A) receptor.
Assessment of affinity and dissociation ability of a newly synthesized 5-HT2 antagonist, AT-1015: comparison with other 5-HT2 antagonists.[Pubmed:11885967]
Jpn J Pharmacol. 2001 Nov;87(3):189-94.
This study investigated the binding affinities of a newly synthesized 5-HT2 antagonist, AT-1015 (N-[2-[4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-piperidino]ethyl]-1-formyl-4-pipe ridinecarboxamide monohydrochloride monohydrate) for [3H]ketanserin bindings to 5-HT2 receptors in the rabbit cerebral cortex membranes using the radioligand binding assay method. The affinity of this compound was also compared with other 5-HT2-selective antagonists such as ketanserin, sarpogrelate, cyproheptadine and ritanserin, and the results showed that AT-1015 has a high pKi value for the 5-HT2 receptor. The rank order of these antagonists are: ritanserin > ketanserin approximately equal to AT-1015 > cyproheptadine approximately equal to sarpogrelate. We also evaluated the dissociation ability (slow or rapid) of AT-1015 in the rabbit cerebral cortex membrane and compared it with other 5-HT2 antagonists using the radioligand binding assay method. The blockade of [3H]ketanserin binding sites in the rabbit cerebral cortex induced by ketanserin and sarpogrelate was readily reversed by washing, whereas the inhibition by AT-1015, cyproheptadine and ritanserin was not readily reversed by washing. The % of control after washing are 76.10% and 49.55% for AT-1015 at 10(-7.5) and 10(-7.0) M, 67.32% and 50.17% for cyproheptadine at 10(7.5) and 10(-7.0) M, and 72.38% and 39.80% for ritanserin at 10(-9.5) and 10(-9.0) M concentrations, respectively. Thus, these findings suggest that AT-1015 has antagonistic properties towards the 5-HT2 receptor and also shows that AT-1015 slowly dissociates from the 5-HT2 receptor, whereas, ketanserin and sarpogrelate dissociate rapidly from the 5-HT2 receptor, which do not correlate with their affinity.
AT-1015, a novel serotonin (5-HT)2 receptor antagonist, blocks vascular and platelet 5-HT2A receptors and prevents the laurate-induced peripheral vascular lesion in rats.[Pubmed:10774780]
J Cardiovasc Pharmacol. 2000 Apr;35(4):523-30.
The serotonin (5-HT2A) antagonistic activities and the protective effect on laurate-induced peripheral vascular lesions of AT-1015, a novel 5-HT2 receptor antagonist, were investigated. In platelet aggregation, AT-1015 selectively inhibited in vitro 5-HT2A receptor-mediated aggregation, and the activity was almost equivalent to that of ketanserin (5-HT2A/2C receptor antagonist) and 100 times more potent than sarpogrelate (5-HT2A receptor antagonist). AT-1015 also inhibited 5-HT2A receptor-mediated aggregation by oral administration in rat, and the dose required for inhibition was equivalent to ketanserin. In a 5-HT-induced vasoconstriction study in rat, AT-1015 slightly reduced maximal contraction and caused a rightward shift of the concentration-response curve (pKB value, 9.5), which was unlike competitive inhibitors such as ketanserin and sarpogrelate (pA2 value, 9.3 and 8.7, respectively). Moreover, the ex vivo inhibitory activity significantly remained after oral administration (1 mg/kg). In the rat peripheral vascular lesion model, AT-1015 (1 mg/kg, p.o.) effectively prevented progression of peripheral lesions, and it was more potent compared with ketanserin, sarpogrelate, and cilostazol. These results suggest that AT-1015 is a potent 5-HT2A receptor antagonist, and its insurmountable antagonism may be relevant to its therapeutic potential in peripheral vascular disease.


