SLIGKV-NH2PAR2 agonist CAS# 190383-13-2 |
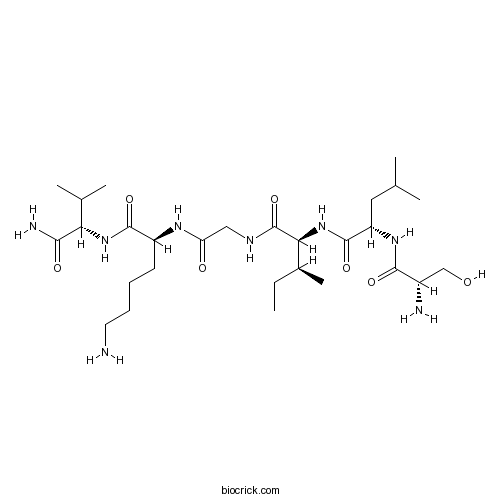
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 190383-13-2 | SDF | Download SDF |
| PubChem ID | 10483914 | Appearance | Powder |
| Formula | C28H54N8O7 | M.Wt | 614.78 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 33.33 mg/mL (54.21 mM; Need ultrasonic) | ||
| Sequence | SLIGKV (Modifications: Val-6 = C-terminal amide) | ||
| Chemical Name | (2S)-6-amino-2-[[2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-2-amino-3-hydroxypropanoyl]amino]-4-methylpentanoyl]amino]-3-methylpentanoyl]amino]acetyl]amino]-N-[(2S)-1-amino-3-methyl-1-oxobutan-2-yl]hexanamide | ||
| SMILES | CCC(C)C(C(=O)NCC(=O)NC(CCCCN)C(=O)NC(C(C)C)C(=O)N)NC(=O)C(CC(C)C)NC(=O)C(CO)N | ||
| Standard InChIKey | HOWDUIVVWDUEED-WAUHAFJUSA-N | ||
| Standard InChI | InChI=1S/C28H54N8O7/c1-7-17(6)23(36-27(42)20(12-15(2)3)34-25(40)18(30)14-37)28(43)32-13-21(38)33-19(10-8-9-11-29)26(41)35-22(16(4)5)24(31)39/h15-20,22-23,37H,7-14,29-30H2,1-6H3,(H2,31,39)(H,32,43)(H,33,38)(H,34,40)(H,35,41)(H,36,42)/t17-,18-,19-,20-,22-,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Protease-activated receptor 2 (PAR2) agonist (Ki = 9.64 μM and IC50 = 10.4 μM). Corresponds to the tethered ligand exposed by trypsin cleavage of PAR-2. Control peptide VKGILS-NH2 also available. |

SLIGKV-NH2 Dilution Calculator

SLIGKV-NH2 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: A protease-activated receptor 2 (PAR2) agonist with an IC50 of 10.4 M.
SLIGKV-NH2 serves as a protease-activated receptor 2 (PAR2) agonist. PARs are a group of G-protein-coupled receptors existing in several cell types. Up to date, four PAR members including PAR1 to 4 have been identified, cloned and designated. PAR2 is expressed in the respiratory and gastrointestinal tracts. It is suggested that the activation of PAR2 is closely correlated with inflammatory evens in various cells and tissues. PAR2 has also been identified to induce protease activation and therefore result in systemic hypotension. [1]
In vitro: It was reported that SLIGKV-NH2 (the PAR2 activating peptide), by inducing express of PAR2, could slightly enhanced mucin secretion by human bronchial epithelial cells in vitro. According to this study, compared to cells treated with a control peptide with reversed amino acid sequence, exposure of cells to SLIGKV-NH2 for 30 mins resulted in a weak but statistically significant increase in mucin secretion at concentrations of 100 and 1000M. In addition, SLIGKV-NH2 was demonstrated to accelerate cell cycle progression and stimulate the growth of HepG2 cells. [1, 2]
In vivo: The ability of PAR2 agonists to induce contractile responses was investigated in vivo. It was found that mouse PAR2 activating (SLIGRL-NH2) and human PAR2 activating (SLIGKV-NH2) peptides triggered a concentration-dependent contractile response in guinea-pig gallbladder. [3]
Clinical trial: PAR2 activating peptide, SLIGKV-NH2, and its reverse-sequence control peptide VKGILS-NH2 were synthesized to verify the hypothesis that in vivo activation of PAR2 in humans would cause vasodilatation. The result of this study showed that, in forearm resistance vessels, SLIGKV-NH2 triggered a dose-dependent dilatation, while VKGILS-NH2 had no significant effect. [4]
References:
[1] Lin KW, Park J, Crews AL, Li YH, Adler KB. Protease-activated receptor-2 (PAR-2) is a weak enhancer of mucin secretion by human bronchial epithelial cells in vitro. Int J Biochem Cell B. 2008. 40: 137988.
[2]Xie L, Zheng Y, Li X, Zhao JY, Chen XY, Chen L, Zhou J, Hai O and Li F. Enhanced proliferation of human hepatoma cells by PAR-2 agonists via the ERK/AP-1 pathway. Oncol Rep. 2012.28: 1665-72.
[3] Tognetto M, Trevisani M, Maggiore B, Navarra G, Turini A, Guerrini R, Bunnett NW, Geppetti P and Harrison S. Evidence that PAR-1 and PAR-2 mediate prostanoid-dependent contraction in isolated guinea-pig gallbladder. Br.J.Pharmacol. 2000.131: 689-94.
[4] Robin J, Kharbanda R, Mclean P, Campbell R, Vallance P. Protease-activated receptor 2–mediated vasodilatation in humans in vivo, role of nitric oxide and prostanoids. Circulation. 2003;107:954-959.
- Orientanol A
Catalog No.:BCN4064
CAS No.:190381-82-9
- 3-Butyryloxytropane
Catalog No.:BCN1924
CAS No.:19038-34-7
- Sarracine N-oxide
Catalog No.:BCN2022
CAS No.:19038-27-8
- 7-Hydroxy-4-Methyl-8-Nitrocoumarin
Catalog No.:BCC9210
CAS No.:19037-69-5
- GR 135531
Catalog No.:BCC6836
CAS No.:190277-13-5
- Bripiodionene
Catalog No.:BCN1831
CAS No.:190265-70-4
- Spiratisanin C
Catalog No.:BCN6975
CAS No.:1902173-22-1
- Spiratisanin B
Catalog No.:BCN6976
CAS No.:1902173-19-6
- Spiratisanin A
Catalog No.:BCN6977
CAS No.:1902173-16-3
- Demethoxyencecalin
Catalog No.:BCN1175
CAS No.:19013-07-1
- Eupatoriochromene
Catalog No.:BCN1174
CAS No.:19013-03-7
- 7-Hydroxy-2,2-dimethylchromene
Catalog No.:BCN7784
CAS No.:19012-97-6
- 17β-Benzoyloxy-androsta-1,4-dien-3-one
Catalog No.:BCC8443
CAS No.:19041-66-8
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- AT 1015
Catalog No.:BCC6194
CAS No.:190508-50-0
- Dioscin
Catalog No.:BCN6273
CAS No.:19057-60-4
- Prosapogenin A
Catalog No.:BCN2582
CAS No.:19057-67-1
- Ac-Gly-OEt
Catalog No.:BCC2944
CAS No.:1906-82-7
- Ro 32-3555
Catalog No.:BCC2377
CAS No.:190648-49-8
- Bepotastine Besilate
Catalog No.:BCC4538
CAS No.:190786-44-8
- Gracillin
Catalog No.:BCN5360
CAS No.:19083-00-2
- Isocupressic acid
Catalog No.:BCN1177
CAS No.:1909-91-7
- Triptocallic acid A
Catalog No.:BCN1176
CAS No.:190906-61-7
- Calystegine C2
Catalog No.:BCN1878
CAS No.:190957-44-9
The effect of exogenous activation of protease-activated receptor 2 on cutaneous vasodilatation and sweating in young males during rest and exercise in the heat.[Pubmed:30377641]
Temperature (Austin). 2018 Sep 6;5(3):257-266.
Protease-activated receptor 2 (PAR2) exists in the endothelial cells of skin vessels and eccrine sweat glands. We evaluated the hypothesis that exogeneous activation of PAR2 augments cutaneous vasodilatation and sweating during rest and exercise in the heat. In 10 young males (23 +/- 5 y), cutaneous vascular conductance (CVC) and sweat rate were measured at four forearm skin sites treated with either 1) lactated Ringer (Control), 2) 0.05 mM, 3) 0.5 mM, or 4) 5 mM SLIGKV-NH2 (PAR2 agonist). Participants initially rested in a semi-recumbent posture under a normothermic ambient condition (25 degrees C) for ~60 min. Thereafter, ambient temperature was increased to 35 degrees C while the participants rested for an additional 60 min. Participants then performed a 50-min bout of cycling (~55% of their pre-determined peak oxygen uptake) followed by a 30-min recovery period. Administration of 5 mM SLIGKV-NH2 increased cutaneous vascular conductance relative to the Control site during normothermic resting (P SLIGKV-NH2 (0.05-5 mM) during rest (33-39%max CVC), end-exercise (68-70%max CVC), and postexercise recovery (49-53%max CVC) in the heat (all P > 0.05). There were no differences in sweat rate between the Control and all SLIGKV-NH2-treated sites throughout the protocol (0.21-0.23, 1.20-1.27, and 0.32-0.33 mgmin(-1)cm(-2) for rest, end-exercise, and postexercise in the heat, respectively, all P > 0.05). We show that while exogeneous PAR2 activation induces cutaneous vasodilatation during normothermic rest, it does not influence the cutaneous blood flow and sweating responses during rest, exercise or recovery in the heat.
Drug repositioning as an effective therapy for protease-activated receptor 2 inhibition.[Pubmed:30370939]
J Cell Biochem. 2018 Oct 29.
Proteinase-activated receptor 2 (PAR-2) is a G protein-coupled receptor activated by both trypsin and a specific agonist peptide, SLIGKV-NH2. It has been linked to various pathologies, including pain and inflammation. Several peptide and peptidomimetic agonizts for PAR-2 have been developed exhibiting high potency and efficacy. However, the number of PAR-2 antagonists is smaller. We screened the Food and Drug Administration library of approved compounds to retrieve novel antagonists for repositioning in the PAR-2 structure. The most efficacious compound bicalutamide bound to the PAR-2 binding groove near the extracellular domain as observed in the in silico studies. Further, it showed reduced Ca(2+) release in trypsin activated cells in a dose-dependent manner. Hence, bicalutamide is a novel and potent PAR-2 antagonist which could be therapeutically useful in blocking multiple pathways diverging from PAR-2 signaling. Further, the novel scaffold of bicalutamide represents a new molecular structure for PAR-2 antagonism and can serve as a basis for further drug development.
Trypsin induces biphasic muscle contraction and relaxation via transient receptor potential vanilloid 1 and neurokinin receptors 1/2 in porcine esophageal body.[Pubmed:28088386]
Eur J Pharmacol. 2017 Feb 15;797:65-74.
Duodenal reflux of fluids containing trypsin relates to refractory gastroesophageal reflux disease (GERD). Esophageal peristalsis and clearance are important factors in GERD pathogenesis. However, the function of trypsin in esophageal body contractility is not fully understood. In this study, effects of trypsin on circular smooth muscle (CSM) and longitudinal smooth muscle (LSM) of the porcine esophageal body were examined. Trypsin elicited a concentration dependent biphasic response, a major contraction and a subsequent relaxation only in CSM. In CSM, contraction occurred at trypsin concentrations of 100nM and relaxation at 1muM. A proteinase-activated receptor (PAR)2 activating peptide, SLIGKV-NH2 (1mM), induced a monophasic contraction. Those responses were unaffected by tetrodotoxin though abolished by the gap junction uncouplers carbenoxolone and octanol. They were also partially inhibited by a transient receptor potential vanilloid type 1 (TRPV1) antagonist and abolished by combination of neurokinin receptor 1 (NK1) and NK2 antagonists, but not by an NK3 antagonist, suggesting a PAR2-TRPV1-substance P pathway in sensory neurons. Substance P (100nM), an agonist for various NK receptors (NK1, NK2 and NK3) with differing affinities, induced significant contraction in CSM, but not in LSM. The contraction was also blocked by the combination of NK1 and NK2 antagonists, but not by the NK3 antagonist. Moreover, substance P-induced contractions were unaffected by the TRPV1 antagonist, but inhibited by a gap junction uncoupler. In conclusion, trypsin induced a biphasic response only in CSM and this was mediated by PAR2, TRPV1 and NK1/2. Gap junctions were indispensable in this tachykinin-induced response.
Activation of protease-activated receptor 2 mediates cutaneous vasodilatation but not sweating: roles of nitric oxide synthase and cyclo-oxygenase.[Pubmed:27981668]
Exp Physiol. 2017 Feb 1;102(2):265-272.
NEW FINDINGS: What is the central question of this study? Protease-activated receptor 2 (PAR2) is located in the endothelial cells of skin vessels and eccrine sweat glands. However, a functional role of PAR2 in the control of cutaneous blood flow and sweating remains to be assessed in humans in vivo. What is the main finding and its importance? Our results demonstrate that in normothermic resting humans in vivo, activation of PAR2 elicits cutaneous vasodilatation partly through nitric oxide synthase-dependent mechanisms, but does not mediate sweating. These results provide important new insights into the physiological significance of PAR2 in human skin. Protease-activated receptor 2 (PAR2) is present in human skin, including keratinocytes, endothelial cells of skin microvessels and eccrine sweat glands. However, whether PAR2 contributes functionally to the regulation of cutaneous blood flow and sweating remains entirely unclear in humans in vivo. We hypothesized that activation of PAR2 directly stimulates cutaneous vasodilatation and sweating via actions of nitric oxide synthase (NOS) and cyclo-oxygenase (COX). In 12 physically active young men (29 +/- 5 years old), cutaneous vascular conductance (CVC) and sweat rate were measured at four intradermal microdialysis forearm skin sites that were treated with the following: (i) lactated Ringer's solution (control); (ii) 10 mm N(G) -nitro-l-arginine (NOS inhibitor); (iii) 10 mm ketorolac (COX inhibitor); or (iv) a combination of both inhibitors. At all sites, a PAR2 agonist (SLIGKV-NH2 ) was co-administered in a dose-dependent fashion (0.06, 0.18, 0.55, 1.66 and 5 mm, each for 25 min). The highest dose of SLIGKV-NH2 (5 mm) increased CVC from baseline at the control site (P 0.05). No increase in sweat rate was measured at any administered dose of SLIGKV-NH2 (all P > 0.05). We show that in normothermic resting humans in vivo, PAR2 activation does not increase sweat rate, whereas it does modulate cutaneous vasodilatation through NOS-dependent mechanisms.
Hypersensitivity Induced by Activation of Spinal Cord PAR2 Receptors Is Partially Mediated by TRPV1 Receptors.[Pubmed:27755539]
PLoS One. 2016 Oct 18;11(10):e0163991.
Protease-activated receptors 2 (PAR2) and transient receptor potential vanilloid 1 (TRPV1) receptors in the peripheral nerve endings are implicated in the development of increased sensitivity to mechanical and thermal stimuli, especially during inflammatory states. Both PAR2 and TRPV1 receptors are co-expressed in nociceptive dorsal root ganglion (DRG) neurons on their peripheral endings and also on presynaptic endings in the spinal cord dorsal horn. However, the modulation of nociceptive synaptic transmission in the superficial dorsal horn after activation of PAR2 and their functional coupling with TRPV1 is not clear. To investigate the role of spinal PAR2 activation on nociceptive modulation, intrathecal drug application was used in behavioural experiments and patch-clamp recordings of spontaneous, miniature and dorsal root stimulation-evoked excitatory postsynaptic currents (sEPSCs, mEPSCs, eEPSCs) were performed on superficial dorsal horn neurons in acute rat spinal cord slices. Intrathecal application of PAR2 activating peptide SLIGKV-NH2 induced thermal hyperalgesia, which was prevented by pretreatment with TRPV1 antagonist SB 366791 and was reduced by protein kinases inhibitor staurosporine. Patch-clamp experiments revealed robust decrease of mEPSC frequency (62.8 +/- 4.9%), increase of sEPSC frequency (127.0 +/- 5.9%) and eEPSC amplitude (126.9 +/- 12.0%) in dorsal horn neurons after acute SLIGKV-NH2 application. All these EPSC changes, induced by PAR2 activation, were prevented by SB 366791 and staurosporine pretreatment. Our results demonstrate an important role of spinal PAR2 receptors in modulation of nociceptive transmission in the spinal cord dorsal horn at least partially mediated by activation of presynaptic TRPV1 receptors. The functional coupling between the PAR2 and TRPV1 receptors on the central branches of DRG neurons may be important especially during different pathological states when it may enhance pain perception.
Skin Barrier Recovery by Protease-Activated Receptor-2 Antagonist Lobaric Acid.[Pubmed:27169822]
Biomol Ther (Seoul). 2016 Sep 1;24(5):529-35.
Atopic dermatitis (AD) results from gene and environment interactions that lead to a range of immunological abnormalities and breakdown of the skin barrier. Protease-activated receptor 2 (PAR2) belongs to a family of G-protein coupled receptors and is expressed in suprabasal layers of the epidermis. PAR2 is activated by both trypsin and a specific agonist peptide, SLIGKV-NH(2) and is involved in both epidermal permeability barrier homeostasis and epithelial inflammation. In this study, we investigated the effect of lobaric acid on inflammation, keratinocyte differentiation, and recovery of the skin barrier in hairless mice. Lobaric acid blocked trypsin-induced and SLIGKV-NH2-induced PAR2 activation resulting in decreased mobilization of intracellular Ca(2)(+) in HaCaT keratinocytes. Lobaric acid reduced expression of interleukin-8 induced by SLIGKV-NH(2) and thymus and activation regulated chemokine (TARC) induced by tumor necrosis factor-a (TNF-alpha) and IFN-gamma in HaCaT keratinocytes. Lobaric acid also blocked SLIGKV-NH(2)-induced activation of ERK, which is a downstream signal of PAR2 in normal human keratinocytes (NHEKs). Treatment with SLIGKV-NH(2) downregulated expression of involucrin, a differentiation marker protein in HaCaT keratinocytes, and upregulated expression of involucrin, transglutamase1 and filaggrin in NHEKs. However, lobaric acid antagonized the effect of SLIGKV-NH(2) in HaCaT keratinocytes and NHEKs. Topical application of lobaric acid accelerated barrier recovery kinetics in a SKH-1 hairless mouse model. These results suggested that lobaric acid is a PAR2 antagonist and could be a possible therapeutic agent for atopic dermatitis.
Mixed exposure to bacterial lipopolysaccharide and seafood proteases augments inflammatory signalling in an airway epithelial cell model (A549).[Pubmed:26149191]
Toxicol Ind Health. 2016 Nov;32(11):1866-1874.
Seafood industry workers exhibit increased prevalence of respiratory symptoms due to exposure to bioaerosols containing a mixture of bioactive agents. In this study, a human pulmonary epithelial cell model (A549) was exposed to mixtures of bacterial lipopolysaccharide (LPS) and protease-activated receptor-2 (PAR-2) agonists H-Ser-Leu-Ile-Gly-Lys-Val-NH2 (SLIGKV-NH2), purified salmon ( Salmo salar) trypsin or purified king crab ( Paralithodes camtschaticus) trypsin. The inflammatory response was measured based on nuclear factor-kappa B (NF-kappaB) activation of transcription in a luciferase reporter gene assay and interleukin 8 (IL-8) secretion in an enzyme-linked immunosorbent assay. We observed that mixtures of SLIGKV-NH2 or trypsins with LPS augmented the activation of NF-kappaB and secretion of IL-8. The effect on IL-8 secretion was synergistic when both trypsins and LPS were used in the lower concentration range. The results demonstrate that exposure to mixtures of agents that are relevant to seafood industry workplaces may lead to increased inflammatory signalling compared with exposure to the individual agents alone. Furthermore, the results indicate that synergism may occur with the combined exposure to seafood trypsins and LPS and is most likely to occur when exposure to either agent is low.
Proteinase-activated receptor-1 (PAR1) and PAR2 mediate relaxation of guinea pig internal anal sphincter.[Pubmed:24631471]
Regul Pept. 2014 Feb 10;189:46-50.
Activation of proteinase-activated receptor-1 (PAR1) and PAR2 stimulates contraction of the rat but relaxation of the guinea pig colon. The aim of the present study was to investigate PAR effects on internal anal sphincter (IAS) motility. We measured relaxation of isolated muscle strips from the guinea pig IAS caused by PAR agonists using isometric transducers. Reverse transcription polymerase chain reaction (RT-PCR) was performed to determine the existence of PAR. In the IAS, thrombin and PAR1 peptide agonists TFLLR-NH2 and SFLLRN-NH2 evoked moderate to marked relaxation in a concentration-dependent manner. In addition, trypsin and PAR2 peptide agonists 2-furoyl-LIGRLO-NH2, SLIGRL-NH2 and SLIGKV-NH2 produced relaxation. In contrast, both PAR1 and PAR2 inactive control peptides did not elicit relaxation. Furthermore, the selective PAR1 antagonist vorapaxar and PAR2 antagonist GB 83 specifically inhibited thrombin and trypsin-induced relaxations, respectively. RT-PCR revealed the presence of PAR1 and PAR2 in the IAS. This indicates that PAR1 and PAR2 mediate the IAS relaxation. The relaxant responses of TFLLR-NH2 and trypsin were attenuated by N(omega)-Nitro-L-arginine (L-NNA), indicating involvement of NO. These responses were not affected by tetrodotoxin, implying that the PAR effects are not neurally mediated. On the other hand, PAR4 agonists GYPGKF-NH2, GYPGQV-NH2 and AYPGKF-NH2 did not cause relaxation or contraction, suggesting that PAR4 is not involved in the sphincter motility. Taken together, these results demonstrate that both PAR1 and PAR2 mediate relaxation of the guinea pig IAS through the NO pathway. PAR1 and PAR2 may regulate IAS tone and might be potential therapeutic targets for anal motility disorders.
Protease activated receptor-2 expression and function in asthmatic bronchial smooth muscle.[Pubmed:24551046]
PLoS One. 2014 Feb 13;9(2):e86945.
Asthmatic bronchial smooth muscle (BSM) is characterized by structural remodeling associated with mast cell infiltration displaying features of chronic degranulation. Mast cell-derived tryptase can activate protease activated receptor type-2 (PAR-2) of BSM cells. The aims of the present study were (i) to evaluate the expression of PAR-2 in both asthmatic and non asthmatic BSM cells and, (ii) to analyze the effect of prolonged stimulation of PAR-2 in asthmatic BSM cells on cell signaling and proliferation. BSM cells were obtained from both 33 control subjects and 22 asthmatic patients. PAR-2 expression was assessed by flow cytometry, western blot and quantitative RT-PCR. Calcium response, transduction pathways and proliferation were evaluated before and following PAR-2 stimulation by SLIGKV-NH2 or trypsin for 1 to 3 days. Asthmatic BSM cells expressed higher basal levels of functional PAR-2 compared to controls in terms of mRNA, protein expression and calcium response. When PAR-2 expression was increased by means of lentivirus in control BSM cells to a level similar to that of asthmatic cells, PAR-2-induced calcium response was then similar in both types of cell. However, repeated PAR-2 stimulations increased the proliferation of asthmatic BSM cells but not that of control BSM cells even following lentiviral over-expression of PAR-2. Such an increased proliferation was related to an increased phosphorylation of ERK in asthmatic BSM cells. In conclusion, we have demonstrated that asthmatic BSM cells express increased baseline levels of functional PAR-2. This higher basal level of PAR-2 accounts for the increased calcium response to PAR-2 stimulation, whereas the increased proliferation to repeated PAR-2 stimulation is related to increased ERK phosphorylation.
Impact on inflammation and recovery of skin barrier by nordihydroguaiaretic Acid as a protease-activated receptor 2 antagonist.[Pubmed:24009835]
Biomol Ther (Seoul). 2012 Sep;20(5):463-9.
Atopic dermatitis is a chronic, inflammatory disease of the skin with increased transepidermal water loss. Both an abnormal inflammatory response and a defective skin barrier are known to be involved in the pathogenesis of atopic dermatitis. Protease activated receptor 2 (PAR2) belongs to a family of G-protein coupled receptors and is activated by both trypsin and a specific agonist peptide, SLIGKV-NH2. PAR2 is expressed in suprabasal layers of the epidermis and regulates inflammatory responses and barrier homeostasis. In this study, we show that nordihydroguaiaretic acid (NDGA) inhibits the PAR2-mediated signal pathway and plays a role in skin barrier recovery in atopic dermatitis. Specifically, NDGA reduces the mobilization of intracellular Ca(2+) in HaCaT keratinocytes by down-regulating inflammatory mediators, such as interleukin-8, thymus and activation-regulated chemokine and intercellular cell adhesion molecule-1 in HaCaT keratinocytes. Also, NDGA decreases the protein expression of involucrin, a differentiation maker of keratinocyte, in both HaCaT keratinocytes and normal human epidermal keratinocytes. We examined NDGA-recovered skin barrier in atopic dermatitis by using an oxazolone-induced atopic dermatitis model in hairless mice. Topical application of NDGA produced an increase in transepidermal water loss recovery and a decrease in serum IgE level, without weight loss. Accordingly, we suggest that NDGA acts as a PAR2 antagonist and may be a possible therapeutic agent for atopic dermatitis.
Protease-activated receptor-2 induces proinflammatory cytokine and chemokine gene expression in canine keratinocytes.[Pubmed:23465358]
Vet Immunol Immunopathol. 2013 May 15;153(1-2):17-25.
Although the molecular basis of the allergenicity remains to be fully elucidated, the ability of allergens to elicit allergic responses is at least partly attributed to their proteolytic activity. Protease-activated receptor-2 (PAR-2) is a G protein-coupled receptor that is activated by site-specific proteolysis by serine proteases and is known to mediate inflammatory processes in various tissues. In this study, we investigated the effects of trypsin, a major serine protease, and a human PAR-2 agonist peptide (SLIGKV-NH2) on proinflammatory cytokine and chemokine gene expression in the canine keratinocyte cell line CPEK. The expression of PAR-2 mRNA and protein in CPEK cells was detected by RT-PCR and Western blotting, respectively. The localization of PAR-2 in CPEK was examined by immunofluorescence. The mRNA expression levels of proinflammatory cytokines and chemokines were quantified by real-time RT-PCR. The free intracellular Ca(2+) concentration was measured using the Ca(2+)-sensitive fluorescent dye. CPEK cells constitutively expressed PAR-2 mRNA and protein. Stimulation of CPEK cells with trypsin induced significant upregulation of the mRNA expression levels of tumor necrosis factor alpha (TNF-alpha, P<0.05), granulocyte-macrophage colony-stimulating factor (GM-CSF, P<0.01), thymus and activation regulated chemokine (TARC/CCL17, P<0.01), and interleukin 8 (IL-8/CXCL8, P<0.01). Similarly, the PAR-2 agonist peptide increased the mRNA expression levels of TNF-alpha (P<0.05), GM-CSF (P<0.05), TARC/CCL17 (P<0.05), and IL-8/CXCL8 (P<0.05) in CPEK cells. Both trypsin and the PAR-2 agonist peptide increased the intracellular Ca(2+) concentration and PAR-2 internalization. These results suggest that PAR-2 activation can augment inflammatory cytokine and chemokine expression in canine keratinocytes, and it may initiate allergic inflammation through the proteolytic activity of allergens in canine atopic dermatitis.
PAR2-induced inflammatory responses in human kidney tubular epithelial cells.[Pubmed:23283995]
Am J Physiol Renal Physiol. 2013 Mar 15;304(6):F737-50.
Protease-activated receptor-2 (PAR2) is a G protein-coupled receptor abundantly expressed in the kidney. The aim of this study was to profile inflammatory gene and protein expression induced by PAR2 activation in human kidney tubular epithelial cells (HTEC). A novel PAR2 antagonist, GB88, was used to confirm agonist specificity. Intracellular Ca(2+) (iCa(2+)) mobilization, confocal microscopy, gene expression profiling, qRTPCR, and protein expression were used to characterize PAR2 activation. PAR2 induced a pronounced increase in iCa(2+) concentration that was blocked by the PAR2 antagonist. Treatment with SLIGKV-NH2 at the apical or basolateral cell surface for 5 h induced expression of a range of inflammatory genes by greater than fourfold, including IL-1beta, TRAF1, IL-6, and MMP-1, as assessed by cDNA microarray and qRTPCR analysis. Using antibody arrays, GM-CSF, ICAM-1, TNF-alpha, MMP-1, and MMP-10 were among the induced proteins secreted. Cytokine-specific ELISAs identified three- to sixfold increases in GM-CSF, IL-6, IL-8, and TNF-alpha, which were blocked by GB88 and protein kinase C inhibitors. Treatment of cells at the basolateral surface induced more potent inflammatory responses, with release of MCP-1 and fibronectin to the apical and basolateral compartments; apical treatment only increased secretion of these factors to the apical compartment. PAR2 activation at the basolateral surface dramatically reduced transepithelial electrical resistance (TEER) whereas apical treatment had no effect. There was very little leakage (<5%) of peptides across the cell monolayer (liquid chromatography-mass spectrometry). In summary, SLIGKV-NH2 induced robust proinflammatory responses in HTEC that were antagonized by GB88. These results suggest that PAR2 antagonists could be useful disease-modifying, anti-inflammatory agents in kidney disease.
Trypsin impaired epithelial barrier function and induced IL-8 secretion through basolateral PAR-2: a lesson from a stratified squamous epithelial model.[Pubmed:22997195]
Am J Physiol Gastrointest Liver Physiol. 2012 Nov 15;303(10):G1105-12.
Immune-mediated injury by the protease-activated receptor-2-interleukin-8 (PAR-2-IL8) pathway may underlie the development of gastroesophageal reflux disease (GERD). However, the localization of PAR-2 and the mechanism of PAR-2 activation remain unclear. This study aimed to address these questions on an esophageal stratified squamous epithelial model and in the human esophageal mucosa of GERD patients. Normal human esophageal epithelial cells were cultured with the air-liquid interface system to establish the model. SLIGKV-NH2 (PAR-2 synthetic agonist), trypsin (PAR-2 natural activator), and weak acid (pH 4, 5, and 6) were added to either the apical or basolateral compartment to evaluate their effects on transepithelial electrical resistance (TEER) and IL-8 production. PAR-2 localization was examined both in the cell model and biopsies from GERD patients by immunohistochemistry. Apical trypsin stimulation induced IL-8 accompanied by decreased TEER in vitro, whereas the effective concentration from the basolateral side was 10 times lower. SLIGKV-NH2 from basolateral but not apical stimulation induced IL-8 production. Apical weak acid stimulation did not influence TEER or IL-8 production. Immunohistochemistry showed intense reactivity of PAR-2 in the basal and suprabasal layers after stimulation with trypsin. A similar PAR-2 reactivity that was mainly located at the basal and suprabasal layers was detected in GERD patients. In conclusion, the activation of the PAR-2-IL-8 pathway probably occurred at the basal and suprabasal layers, while the esophageal epithelial barrier may influence the activation of PAR-2. Under proton pump inhibitor therapy, refluxed trypsin may remain active and be a potential agent in the pathogenesis of refractory GERD.
Binding of a highly potent protease-activated receptor-2 (PAR2) activating peptide, [3H]2-furoyl-LIGRL-NH2, to human PAR2.[Pubmed:15765104]
Br J Pharmacol. 2005 May;145(2):255-63.
1 To determine the binding characteristics of a highly potent agonist for protease-activated receptor-2 (PAR2), 2-furoyl-Leu-Ile-Gly-Arg-Leu-amide (2-furoyl-LIGRL-NH(2)), whole-cell binding assays were performed utilising a radioactive ligand, [(3)H]2-furoyl-LIGRL-NH(2). 2 Specific binding of [(3)H]2-furoyl-LIGRL-NH(2) was observed in NCTC2544 cells, dependent upon PAR2 expression, and competitively displaced by the addition of unlabeled PAR2 agonists. Scatchard analysis of specific saturation binding suggested a single binding site, with K(d) of 122+/-26.1 nM and a corresponding B(max) of 180+/-6 f mol in 3.0 x 10(5) cells. 3 The relative binding affinities of a series of modified PAR2 agonist peptides obtained from competition studies paralleled their relative EC(50) values for Ca(2+) mobilisation assays, indicating improved binding affinities by substitution with 2-furoyl at the N-terminus serine. 4 Pretreatment of cells with trypsin reduced specific binding of [(3)H]2-furoyl-LIGRL-NH(2), demonstrating direct competition between the synthetic agonist peptide and the proteolytically revealed tethered ligand for the binding site of the receptor. 5 In HCT-15 cells endogenously expressing PAR2, the binding of [(3)H]2-furoyl-LIGRL-NH(2) was displaced by addition of unlabeled ligands, Ser-Leu-Ile-Gly-Lys-Val (SLIGKV-OH) or 2-furoyl-LIGRL-NH(2). The relative binding affinity of 2-furoyl-LIGRL-NH(2) to SLIGKV-OH was comparable to its relative EC(50) value for Ca(2+) mobilisation assays. 6 The binding assay was successfully performed in monolayers of PAR2 expressing NCTC2544 and human umbilical vein endothelial cells (HUVEC), in 96- and 24-well plate formats, respectively. 7 These studies indicate that [(3)H]2-furoyl-LIGRL-NH(2) binds to human PAR2 at its ligand-binding site. The use of this radioligand will be valuable for characterising chemicals that interact to PAR2.
International Union of Pharmacology. XXVIII. Proteinase-activated receptors.[Pubmed:12037136]
Pharmacol Rev. 2002 Jun;54(2):203-17.
Proteinase-activated receptors (PARs) represent a unique subclass of G-protein-coupled receptors of which four family members have now been cloned from a number of species. The novel mechanism of receptor activation involves the proteolytic unmasking of a cryptic N-terminal receptor sequence that, remaining tethered, binds to and triggers receptor function. In addition, short (five to six amino acids) synthetic peptides, based on the proteolytically revealed motif, can activate PARs without the unmasking of the tethered ligand. This article summarizes the experiments leading to the pharmacological characterization and cloning of the four PAR family members and provides a rationale for their designation by the acronym "PAR". The ability to distinguish among the PARs pharmacologically 1) with selective proteinase activators, 2) with receptor-selective peptide agonists, and 3) with peptide and nonpeptide antagonists is discussed, as are the molecular mechanisms of receptor activation and desensitization/internalization. Finally, the potential physiological roles of the PARs, which are widely distributed in many organs in the settings of tissue injury, repair, and remodeling, including embryogenesis and oncogenesis are discussed, and the newly appreciated roles of proteinases as signaling molecules that can act as either functional agonists or antagonists are highlighted.
Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2.[Pubmed:8615752]
Biochem J. 1996 Mar 15;314 ( Pt 3):1009-16.
We used PCR to amplify proteinase activated receptor-2 (PAR-2) from human kidney cDNA. The open reading frame comprised 1191 bp and encoded a protein of 397 residues with 83% identity with mouse PAR-2. In KNRK cells (a line of kirsten murine sarcoma virus-transformed rat kidney epithelial cells) transfected with this cDNA, trypsin and activating peptide (AP) corresponding to the tethered ligand exposed by trypsin cleavage (SLIGKV-NH2) induced a prompt increase in cytosolic calcium ion concentration ([Ca2+]i). Human PAR-2 (hPAR-2) resided both on the plasma membrane and in the Golgi apparatus. hPAR-2 mRNA was highly expressed in human pancreas, kidney, colon, liver and small intestine, and by A549 lung and SW480 colon adenocarcinoma cells. Hybridization in situ revealed high expression in intestinal epithelial cells throughout the gut. Trypsin and AP stimulated an increase in [Ca2+]i in a rat intestinal epithelial cell line (hBRIE 380) and stimulated amylase secretion in isolated pancreatic acini. In A549 cells, which also responded to trypsin and AP with mobilization of cytosolic Ca2+, AP inhibited colony formation. Thus PAR-2 may serve as a trypsin sensor in the gut. Its expression by cells and tissues not normally exposed to pancreatic trypsin suggests that other proteases could serve as physiological activators.


