DemethoxyencecalinCAS# 19013-07-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

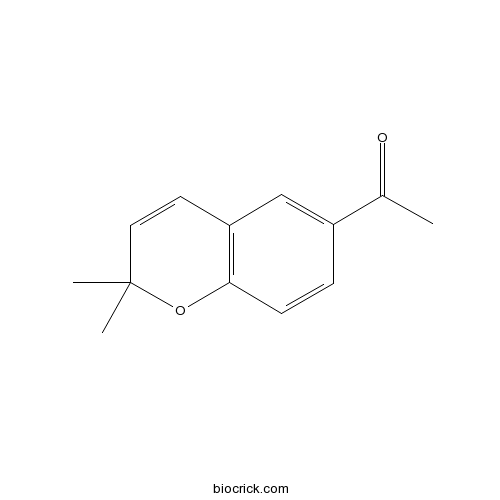
| Cas No. | 19013-07-1 | SDF | Download SDF |
| PubChem ID | 177040 | Appearance | Powder |
| Formula | C13H14O2 | M.Wt | 202.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(2,2-dimethylchromen-6-yl)ethanone | ||
| SMILES | CC(=O)C1=CC2=C(C=C1)OC(C=C2)(C)C | ||
| Standard InChIKey | ZAJTXVHECZCXLH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H14O2/c1-9(14)10-4-5-12-11(8-10)6-7-13(2,3)15-12/h4-8H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Antifungal activity of a new phenolic compound from capitulum of a head rot-resistant sunflower genotype.[Pubmed: 18034282]J Chem Ecol. 2007 Dec;33(12):2245-53.
Accumulation and Biotransformation of Chromenes and Benzofurans in a Cell Suspension Culture of Ageratina adenophora.[Pubmed: 17269074]Planta Med. 1987 Oct;53(5):488-92.A cell suspension culture of AGERATINA ADENOPHORA was shown to yield several novel chromene and benzofuran derivatives in minute amounts that were different to the compounds found in seedlings of the same species. The structure elucidation of the new compounds is described.
|
| Structure Identification | Planta Med. 1986 Oct;(5):349-51.[Demethoxyencecalin and Thymol derivatives from Arnica sachalinensis1.].[Pubmed: 17345336]
|

Demethoxyencecalin Dilution Calculator

Demethoxyencecalin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.9432 mL | 24.7158 mL | 49.4315 mL | 98.8631 mL | 123.5788 mL |
| 5 mM | 0.9886 mL | 4.9432 mL | 9.8863 mL | 19.7726 mL | 24.7158 mL |
| 10 mM | 0.4943 mL | 2.4716 mL | 4.9432 mL | 9.8863 mL | 12.3579 mL |
| 50 mM | 0.0989 mL | 0.4943 mL | 0.9886 mL | 1.9773 mL | 2.4716 mL |
| 100 mM | 0.0494 mL | 0.2472 mL | 0.4943 mL | 0.9886 mL | 1.2358 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Eupatoriochromene
Catalog No.:BCN1174
CAS No.:19013-03-7
- 7-Hydroxy-2,2-dimethylchromene
Catalog No.:BCN7784
CAS No.:19012-97-6
- N-Acetyl-O-phosphono-Tyr-Glu Dipentylamide
Catalog No.:BCC5855
CAS No.:190078-50-3
- Firocoxib
Catalog No.:BCC5498
CAS No.:189954-96-9
- Mesopram
Catalog No.:BCC7549
CAS No.:189940-24-7
- Palmitoylisopropylamide
Catalog No.:BCC7187
CAS No.:189939-61-5
- 3-O-Feruloylquinic acid
Catalog No.:BCN3353
CAS No.:1899-29-2
- 8-Amino-2-methylquinoline
Catalog No.:BCC8782
CAS No.:18978-78-4
- Ro 10-5824 dihydrochloride
Catalog No.:BCC7330
CAS No.:189744-94-3
- Akuammiline
Catalog No.:BCN4772
CAS No.:1897-26-3
- 6,4'-Dihydroxy-7-methoxyflavanone
Catalog No.:BCN7797
CAS No.:189689-32-5
- trans-4-phenylbut-3-en-2-one
Catalog No.:BCN3805
CAS No.:1896-62-4
- Spiratisanin A
Catalog No.:BCN6977
CAS No.:1902173-16-3
- Spiratisanin B
Catalog No.:BCN6976
CAS No.:1902173-19-6
- Spiratisanin C
Catalog No.:BCN6975
CAS No.:1902173-22-1
- Bripiodionene
Catalog No.:BCN1831
CAS No.:190265-70-4
- GR 135531
Catalog No.:BCC6836
CAS No.:190277-13-5
- 7-Hydroxy-4-Methyl-8-Nitrocoumarin
Catalog No.:BCC9210
CAS No.:19037-69-5
- Sarracine N-oxide
Catalog No.:BCN2022
CAS No.:19038-27-8
- 3-Butyryloxytropane
Catalog No.:BCN1924
CAS No.:19038-34-7
- Orientanol A
Catalog No.:BCN4064
CAS No.:190381-82-9
- SLIGKV-NH2
Catalog No.:BCC3959
CAS No.:190383-13-2
- 17β-Benzoyloxy-androsta-1,4-dien-3-one
Catalog No.:BCC8443
CAS No.:19041-66-8
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
[Demethoxyencecalin and Thymol derivatives from Arnica sachalinensis1.].[Pubmed:17345336]
Planta Med. 1986 Oct;(5):349-51.
From the flowers of ARNICA SACHALINENSIS the chromene desmethoxyencecalin, 10-acetoxy-8,9-epoxy-3- O-isobutyrylthymol, and 10-acetoxy-8-hydroxy-9-isobutyryloxythymol were isolated and their structures established by mass spectrometry (13)C-, and (1)H-NMR spectroscopy.
Antifungal activity of a new phenolic compound from capitulum of a head rot-resistant sunflower genotype.[Pubmed:18034282]
J Chem Ecol. 2007 Dec;33(12):2245-53.
In a previous study, we observed that bract and corolla extracts from a Sclerotinia sclerotiorum-resistant sunflower contained high amounts of the known coumarins scopoletin, scopolin, and ayapin. There was a correlation between coumarin concentration and disease resistance. Thin layer chromatography showed higher concentrations of three other compounds in the resistant genotype when compared to the susceptible. A bioassay-directed purification that used column chromatography and HPLC allowed the isolation of a new compound, 3-acetyl-4-acetoxyacetophenone, and known compounds, Demethoxyencecalin and 3-acetyl-4-hydroxyacetophenone. Structures were assigned from spectral data, and bioactivities were characterized by in vitro bioassays against S. sclerotiorum. The new compound, 3-acetyl-4-acetoxyacetophenone, had an antifungal activity similar to the coumarin ayapin, previously described as a potent Sclerotinia inhibitor. The speed and simplicity by which these compounds can be detected make them suitable for use in screening procedures that may identify genotypes with valuable levels of resistance. A screening of seven sunflower genotypes in a field experiment showed a correlation between these compounds and resistance to Sclerotinia.
Accumulation and Biotransformation of Chromenes and Benzofurans in a Cell Suspension Culture of Ageratina adenophora.[Pubmed:17269074]
Planta Med. 1987 Oct;53(5):488-92.
A cell suspension culture of AGERATINA ADENOPHORA was shown to yield several novel chromene and benzofuran derivatives in minute amounts that were different to the compounds found in seedlings of the same species. The structure elucidation of the new compounds is described. When two of the seedling chromenes (Demethoxyencecalin and demethylencecalin) were fed to the cell suspension culture, one biotransformation product each was obtained in high yields (80%) that originated from a hydroxylation at one of the geminal methyl groups of the chromene heterocycle. These products accumulated largely in the growth media even though the presence of cells was necessary for the biotransformations to occur. When the third seedling chromene (encecalin) was fed to the cell auspension culture, no significant biotransformation was noted but several of the benzofurans present as cell culture metabolites showed a significantly increased accumulation in the growth media of the treated cultures. This increased accumulation of benzofurans was found to be inducible also by adding yeast extract to the cell culture. The metabolism of chromenes and bezofurans in the cell suspension culture is discussed.


