3-O-Feruloylquinic acidCAS# 1899-29-2 |
- 5-O-Feruloylquinic acid
Catalog No.:BCN3788
CAS No.:40242-06-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1899-29-2 | SDF | Download SDF |
| PubChem ID | 6451331 | Appearance | Powder |
| Formula | C17H20O9 | M.Wt | 368.3 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
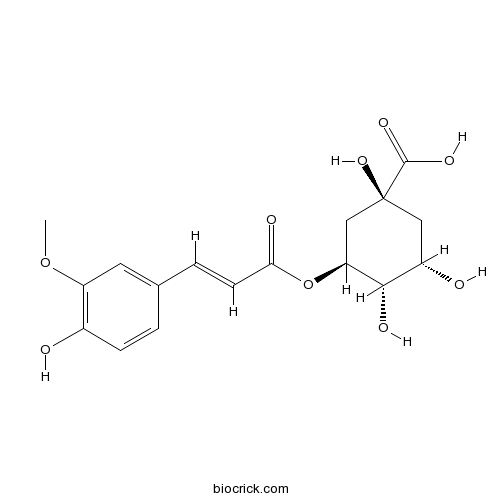
| Chemical Name | (1S,3S,4S,5S)-1,3,4-trihydroxy-5-[(E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoyl]oxycyclohexane-1-carboxylic acid | ||
| SMILES | COC1=C(C=CC(=C1)C=CC(=O)OC2CC(CC(C2O)O)(C(=O)O)O)O | ||
| Standard InChIKey | RAGZUCNPTLULOL-ZLCRQKIYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 3-O-Feruloylquinic acid is a protease inhibitor. 2. 3-O-Feruloylquinic acid exerts moderate inhibitory effect against AIV (H5N1) in vitro. |
| Targets | Antifection | Proteasome |

3-O-Feruloylquinic acid Dilution Calculator

3-O-Feruloylquinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7152 mL | 13.5759 mL | 27.1518 mL | 54.3036 mL | 67.8794 mL |
| 5 mM | 0.543 mL | 2.7152 mL | 5.4304 mL | 10.8607 mL | 13.5759 mL |
| 10 mM | 0.2715 mL | 1.3576 mL | 2.7152 mL | 5.4304 mL | 6.7879 mL |
| 50 mM | 0.0543 mL | 0.2715 mL | 0.543 mL | 1.0861 mL | 1.3576 mL |
| 100 mM | 0.0272 mL | 0.1358 mL | 0.2715 mL | 0.543 mL | 0.6788 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8-Amino-2-methylquinoline
Catalog No.:BCC8782
CAS No.:18978-78-4
- Ro 10-5824 dihydrochloride
Catalog No.:BCC7330
CAS No.:189744-94-3
- Akuammiline
Catalog No.:BCN4772
CAS No.:1897-26-3
- 6,4'-Dihydroxy-7-methoxyflavanone
Catalog No.:BCN7797
CAS No.:189689-32-5
- trans-4-phenylbut-3-en-2-one
Catalog No.:BCN3805
CAS No.:1896-62-4
- Dihydrooroxylin A
Catalog No.:BCN3500
CAS No.:18956-18-8
- 6'-Hydroxy-7'-ethoxybergamottin
Catalog No.:BCC8306
CAS No.:
- Pinostrobin chalcone
Catalog No.:BCN1173
CAS No.:18956-15-5
- Ginsenoside F5
Catalog No.:BCN6419
CAS No.:189513-26-6
- Epothilone D
Catalog No.:BCC1554
CAS No.:189453-10-9
- Boc-Ser(tBu)-OH.DCHA
Catalog No.:BCC3445
CAS No.:18942-50-2
- Boc-D-Phe-OH
Catalog No.:BCC3433
CAS No.:18942-49-9
- Palmitoylisopropylamide
Catalog No.:BCC7187
CAS No.:189939-61-5
- Mesopram
Catalog No.:BCC7549
CAS No.:189940-24-7
- Firocoxib
Catalog No.:BCC5498
CAS No.:189954-96-9
- N-Acetyl-O-phosphono-Tyr-Glu Dipentylamide
Catalog No.:BCC5855
CAS No.:190078-50-3
- 7-Hydroxy-2,2-dimethylchromene
Catalog No.:BCN7784
CAS No.:19012-97-6
- Eupatoriochromene
Catalog No.:BCN1174
CAS No.:19013-03-7
- Demethoxyencecalin
Catalog No.:BCN1175
CAS No.:19013-07-1
- Spiratisanin A
Catalog No.:BCN6977
CAS No.:1902173-16-3
- Spiratisanin B
Catalog No.:BCN6976
CAS No.:1902173-19-6
- Spiratisanin C
Catalog No.:BCN6975
CAS No.:1902173-22-1
- Bripiodionene
Catalog No.:BCN1831
CAS No.:190265-70-4
- GR 135531
Catalog No.:BCC6836
CAS No.:190277-13-5
Isolation and quantification of major chlorogenic acids in three major instant coffee brands and their potential effects on H2O2-induced mitochondrial membrane depolarization and apoptosis in PC-12 cells.[Pubmed:24061869]
Food Funct. 2013 Nov;4(11):1632-8.
Coffee is a most consumed drink worldwide, with potential health effects on several chronic diseases including neuronal degenerative diseases. Chlorogenic acids (CHAs) are phenolic compounds found in coffee and they are reported to have strong antioxidant and anti-inflammatory activities. However, the amounts of CHAs often vary in coffee drinks and their potential effects on ROS-induced neuronal cell death still require more investigation. Therefore, in this paper, major CHAs were isolated from three major instant coffee brands, confirmed and quantified using HPLC and NMR spectroscopic methods. Then, their antioxidant activities and protective effects on H2O2-induced apoptosis in PC-12 cells were investigated using radical scavenging, mitochondrial membrane potential and caspase assays. In the coffee samples, three major CHAs (3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid) and some minor CHAs (3-O-Feruloylquinic acid, 4-O-feruloylquinic acid, 5-O-feruloylquinic acid, 3,5-O-dicaffeoylquinic acid, 3,4-O-dicaffeoylquinic acid, and 4,5-O-dicaffeoylquinic acid) were detected. The three major CHAs were further isolated and their chemical structures were confirmed using NMR spectroscopic techniques. Also, the amounts of the three major CHAs were individually quantified using a HPLC method. At the concentration of 10 muM, all three major CHAs quenched DPPH and/or xanthine oxidase-generated radical species by 21-51% (P < 0.014). They also inhibited H2O2-induced mitochondrial membrane depolarization and caspase-9 activation by 27% (P < 0.034) and 50% (P < 0.05), respectively. This study suggests that the major CHAs found in coffee are likely to be potent antioxidant compounds able to quench radical species as well as inhibit H2O2-induced apoptosis via suppressing mitochondrial membrane depolarization and caspase-9 activation in the cells.
[Chemical constituents of the roots of Macleaya microcarpa and activation efficacy of benzophenanthridine alkaloids for the transcription of xbp1 gene].[Pubmed:25975030]
Yao Xue Xue Bao. 2015 Feb;50(2):207-10.
Ongoing study on the chemical constituents of the roots of Macleaya microcarpa led to the isolation of eight compounds of derivatives of triterpenes and organic acids in addition to some previously identified benzophenanthridines. The eight compounds were identified by spectroscopic methods as well as comparison with literature values as 1-oxo-2, 22 (30)-hopandien-29-oic acid (1), 3-oxo-12-oleanen-30-oic acid (2), 3alpha-hydroxy-12-oleanen-30-oic acid (3), 3beta-hydroxy-12-oleanen-30-oic acid (4), ferulic acid (5), ferulic acid 4-O-beta-D-glucoside (6), 3-O-Feruloylquinic acid (7), and methyl 3-O-feruloylquinate (8). Of which, 1 is a new triterpenoid of hopanes and 2-8 are isolated from M microcarpa for the first time. In order to discover natural active compounds as potential agents of anti-ulcerative colitis (UC), an in vitro drug high-throughput screening model targeted x-box-binding protein 1 (xbp1) was employed to evaluate the activity of the major chemical constituents of M microcarpa. The result confirmed that two dihydrobenzophenanthridines, dihydrosanguinarine (9) and dihydrochelerythrine (10), showed a certain activity on activating the transcription of xbpl, a transcription factor (TF) associated with the occurrence, development, and potential treatment of UC, with their relative activating ratios being 1.76 and 1.77 times, respectively, as compared with control group.


