5-O-Feruloylquinic acidCAS# 40242-06-6 |
- 3-O-Feruloylquinic acid
Catalog No.:BCN3353
CAS No.:1899-29-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40242-06-6 | SDF | Download SDF |
| PubChem ID | 131853509 | Appearance | Powder |
| Formula | C17H20O9 | M.Wt | 368.3 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
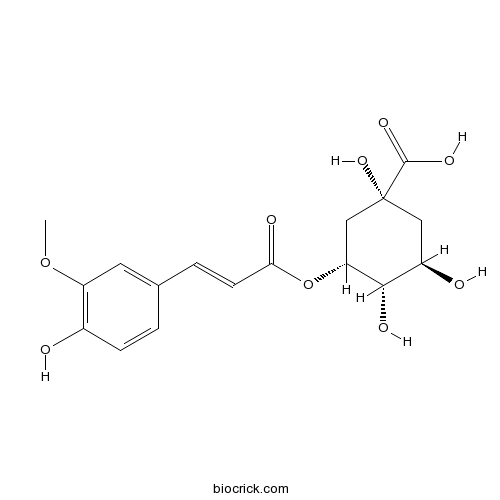
| Chemical Name | (1R,3R,4S,5R)-1,3,4-trihydroxy-5-[3-(4-hydroxy-3-methoxyphenyl)prop-2-enoyloxy]cyclohexane-1-carboxylic acid | ||
| SMILES | COC1=C(C=CC(=C1)C=CC(=O)OC2CC(CC(C2O)O)(C(=O)O)O)O | ||
| Standard InChIKey | RAGZUCNPTLULOL-JSHWQEIDSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-O-feruloylquinic acid is a potent Sirt1 agonist, it is a potential lead compound that can be further tested in drug development process for diseases associated with aging. |
| Targets | Sirt1 |
| In vitro | Investigation of silent information regulator 1 (Sirt1) agonists from Traditional Chinese Medicine.[Pubmed: 23075283]Journal of Biomolecular Structure and Dynamics, 2013, 31(11):1207-1218.Silent information regulator 1 (Sirt1), a class III nicotinamide adenine dinucleotide dependent histone deacetylases, is important in cardioprotection, neuroprotection, metabolic disease, calorie restriction, and diseases associated with aging.
|
| Structure Identification | European Journal of Horticultural Science, 2008, 73(1):20-27.Concentration and composition of flavonol glycosides and phenolic acids in aerial parts of stinging nettle (Urtica dioica L.) are affected by nitrogen fertilization and by harvest time.[Reference: WebLink]
|

5-O-Feruloylquinic acid Dilution Calculator

5-O-Feruloylquinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7152 mL | 13.5759 mL | 27.1518 mL | 54.3036 mL | 67.8794 mL |
| 5 mM | 0.543 mL | 2.7152 mL | 5.4304 mL | 10.8607 mL | 13.5759 mL |
| 10 mM | 0.2715 mL | 1.3576 mL | 2.7152 mL | 5.4304 mL | 6.7879 mL |
| 50 mM | 0.0543 mL | 0.2715 mL | 0.543 mL | 1.0861 mL | 1.3576 mL |
| 100 mM | 0.0272 mL | 0.1358 mL | 0.2715 mL | 0.543 mL | 0.6788 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Hyp-OMe.HCl
Catalog No.:BCC3248
CAS No.:40216-83-9
- H-Orn-OMe.2HCl
Catalog No.:BCC3001
CAS No.:40216-82-8
- Fmoc-β-Homo-Gln(Trt)-OH
Catalog No.:BCC2647
CAS No.:401915-55-7
- Fmoc-β-homo-Arg(Pbf)-OH
Catalog No.:BCC2649
CAS No.:401915-53-5
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- SKA 31
Catalog No.:BCC7743
CAS No.:40172-65-4
- Boc-N-Me-Phe.DCHA
Catalog No.:BCC3348
CAS No.:40163-88-0
- Erucifoline
Catalog No.:BCN2081
CAS No.:40158-95-0
- H-Arg-pNA.2HCl
Catalog No.:BCC2858
CAS No.:40127-11-5
- Ceftaroline fosamil
Catalog No.:BCC5266
CAS No.:400827-46-5
- 20-Hydroxyganoderic acid G
Catalog No.:BCN8231
CAS No.:400604-12-8
- N6-Benzoyladenine
Catalog No.:BCC9075
CAS No.:4005-49-6
- Glycitin
Catalog No.:BCN5895
CAS No.:40246-10-4
- ONO-AE3-208
Catalog No.:BCC1822
CAS No.:402473-54-5
- Acetylcimigenol 3-O-alpha-L-arabinopyranside
Catalog No.:BCN1447
CAS No.:402513-88-6
- DMeOB
Catalog No.:BCC7213
CAS No.:40252-74-2
- NSC 693868
Catalog No.:BCC7208
CAS No.:40254-90-8
- Firategrast
Catalog No.:BCC1575
CAS No.:402567-16-2
- Deacetylcinobufagin
Catalog No.:BCN2720
CAS No.:4026-95-3
- SB 399885 hydrochloride
Catalog No.:BCC7595
CAS No.:402713-81-9
- 7-Methoxyneochamaejasmine A
Catalog No.:BCN3134
CAS No.:402828-38-0
- DR 4485 hydrochloride
Catalog No.:BCC7990
CAS No.:402942-53-4
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- MDL 12330A hydrochloride
Catalog No.:BCC7066
CAS No.:40297-09-4
[Studies on serum pharmacochemistry of effective parts of modified Xiaochaihu Tang for treatment of gastric ulcer].[Pubmed:29751718]
Zhongguo Zhong Yao Za Zhi. 2018 Apr;43(8):1692-1700.
To conduct the studies on serum pharmacochemistry of effective parts of modified Xiaochaihu Tang for treatment of gastric ulcer by using chromatography-mass spectrometry. Absolute ethyl alcohol induced-gastric ulcer model of mouse was used in this study to investigate the pharmacology of modified Xiaochaihu Tang and its effective parts. Both groups could significantly decrease the absolute ethyl alcohol induced-ulcer. Gasphase-mass spectrometry (GC-MS) was used to detect chemical compositions of volatile fractions and the drug components after gastric administration. A total of 63 compounds were identified in extracts, accounting for more than 93% of the all volatile oil, including 23.51% alpha-curcumene, 11.96% zingiberene, 9.56% curzerene, 6.54% beta-sesquinene, 4.77% camphene, and 6 prototype components were also detected in serum for gastric ulcer model. In liquid chromatography-mass spectrometry (LC-MS), a total of 17 compounds were identified in extracts, 6 prototype components and 2 metabolites (3,5-O-Feruloylquinic acid and palmatine) were obtained in serum. In a conclusion, this study provides an important scientific basis for identifying the active ingredients in modified Xiaochaihu Tang, and also helps to reveal the pharmacological effect of modified Xiaochaihu Tang for treatment of gastric ulcer.
Non-invasive Presymptomatic Detection of Cercospora beticola Infection and Identification of Early Metabolic Responses in Sugar Beet.[Pubmed:27713750]
Front Plant Sci. 2016 Sep 22;7:1377.
Cercospora beticola is an economically significant fungal pathogen of sugar beet, and is the causative pathogen of Cercospora leaf spot. Selected host genotypes with contrasting degree of susceptibility to the disease have been exploited to characterize the patterns of metabolite responses to fungal infection, and to devise a pre-symptomatic, non-invasive method of detecting the presence of the pathogen. Sugar beet genotypes were analyzed for metabolite profiles and hyperspectral signatures. Correlation of data matrices from both approaches facilitated identification of candidates for metabolic markers. Hyperspectral imaging was highly predictive with a classification accuracy of 98.5-99.9% in detecting C. beticola. Metabolite analysis revealed metabolites altered by the host as part of a successful defense response: these were L-DOPA, 12-hydroxyjasmonic acid 12-O-beta-D-glucoside, pantothenic acid, and 5-O-Feruloylquinic acid. The accumulation of glucosylvitexin in the resistant cultivar suggests it acts as a constitutively produced protectant. The study establishes a proof-of-concept for an unbiased, presymptomatic and non-invasive detection system for the presence of C. beticola. The test needs to be validated with a larger set of genotypes, to be scalable to the level of a crop improvement program, aiming to speed up the selection for resistant cultivars of sugar beet. Untargeted metabolic profiling is a valuable tool to identify metabolites which correlate with hyperspectral data.
First chemical evaluation and toxicity of Casinga-cheirosa to Balb-c male mice.[Pubmed:24699143]
Molecules. 2014 Apr 2;19(4):3973-87.
Laetia suaveolens, known as "casinga-cheirosa", crude extract EB719 has previously shown cytotoxic activity against prostate cancer and squamous cell carcinoma. For the first time, seven molecules were isolated from its apolar-alpha-tocopherol (1) and sitosterol (2)-and polar-3-O-caffeoylquinic acid (3), 4-O-caffeoylquinic acid (4), 5-O-Feruloylquinic acid (5), hyperoside (6), and isoquercitrin (7)-fractions. Acute toxicity was determined in a two-stage experiment: (1) a reduced number of Balb-c male mice received 5000 mg/kg of EB719 to allow evaluation of general activity and other 27 parameters, plus death, up to the establishment of non-lethal dose (NLD), as well as lethal dose 50% (LD50); (2) NLD was administered and diazepam introduced as reference drug. EB719 showed LD50=178.0 mg/kg, and NLD 156.3 mg/kg. In stage one EB719 did not influence general activity, but provoked impairment in grasp reflexes, tail squeeze and breathing; piloerection and cyanosis were increased. In stage two, alterations occurred in auricular reflex, piloerection and breathing after diazepam administration, but not in response to EB719. Intestinal hemorrhage caused by local bleeding was observed after necropsy, and may be the main cause of animals' death other than a systemic effect of the extract. Although the isolated compounds are biologically and pharmacologically active in both men and animal systems, it is premature to relate their occurrence in EB719 to the observed intestine hemorrhage in mice.
Isolation and quantification of major chlorogenic acids in three major instant coffee brands and their potential effects on H2O2-induced mitochondrial membrane depolarization and apoptosis in PC-12 cells.[Pubmed:24061869]
Food Funct. 2013 Nov;4(11):1632-8.
Coffee is a most consumed drink worldwide, with potential health effects on several chronic diseases including neuronal degenerative diseases. Chlorogenic acids (CHAs) are phenolic compounds found in coffee and they are reported to have strong antioxidant and anti-inflammatory activities. However, the amounts of CHAs often vary in coffee drinks and their potential effects on ROS-induced neuronal cell death still require more investigation. Therefore, in this paper, major CHAs were isolated from three major instant coffee brands, confirmed and quantified using HPLC and NMR spectroscopic methods. Then, their antioxidant activities and protective effects on H2O2-induced apoptosis in PC-12 cells were investigated using radical scavenging, mitochondrial membrane potential and caspase assays. In the coffee samples, three major CHAs (3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid) and some minor CHAs (3-O-feruloylquinic acid, 4-O-feruloylquinic acid, 5-O-Feruloylquinic acid, 3,5-O-dicaffeoylquinic acid, 3,4-O-dicaffeoylquinic acid, and 4,5-O-dicaffeoylquinic acid) were detected. The three major CHAs were further isolated and their chemical structures were confirmed using NMR spectroscopic techniques. Also, the amounts of the three major CHAs were individually quantified using a HPLC method. At the concentration of 10 muM, all three major CHAs quenched DPPH and/or xanthine oxidase-generated radical species by 21-51% (P < 0.014). They also inhibited H2O2-induced mitochondrial membrane depolarization and caspase-9 activation by 27% (P < 0.034) and 50% (P < 0.05), respectively. This study suggests that the major CHAs found in coffee are likely to be potent antioxidant compounds able to quench radical species as well as inhibit H2O2-induced apoptosis via suppressing mitochondrial membrane depolarization and caspase-9 activation in the cells.
Investigation of silent information regulator 1 (Sirt1) agonists from Traditional Chinese Medicine.[Pubmed:23075283]
J Biomol Struct Dyn. 2013;31(11):1207-18.
Silent information regulator 1 (Sirt1), a class III nicotinamide adenine dinucleotide dependent histone deacetylases, is important in cardioprotection, neuroprotection, metabolic disease, calorie restriction, and diseases associated with aging. Traditional Chinese Medicine (TCM) compounds from TCM Database@Taiwan ( http://tcm.cmu.edu.tw/ ) were employed for screening potent Sirt1 agonists, and molecular dynamics (MD) simulation was implemented to simulate ligand optimum docking poses and protein structure under dynamic conditions. TCM compounds such as (S)-tryptophan-betaxanthin, 5-O-Feruloylquinic acid, and RosA exhibited good binding affinity across different computational methods, and their drug-like potential were validated by MD simulation. Docking poses indicate that the carboxylic group of the three candidates generated H-bonds with residues in the protein chain from Ser441 to Lys444 and formed H-bond, pi-cation interactions, or hydrophobic contacts with Phe297 and key active residue, His363. During MD, stable pi-cation interactions with residues Phe273 or Arg274 were formed by (S)-tryptophan-betaxanthin and RosA. All candidates were anchored to His363 by stable pi- or H-bonds. Hence, we propose (S)-tryptophan-betaxanthin, 5-O-Feruloylquinic acid, and RosA as potential lead compounds that can be further tested in drug development process for diseases associated with aging An animated interactive 3D complement (I3DC) is available in Proteopedia at http://proteopedia.org/w/Journal:JBSD:28.
First chemical synthesis and in vitro characterization of the potential human metabolites 5-o-feruloylquinic acid 4'-sulfate and 4'-O-glucuronide.[Pubmed:21417257]
J Agric Food Chem. 2011 May 25;59(10):5671-6.
Feruloylquinic acids are a major class of biologically active phenolic antioxidants in coffee beans, but their metabolic fate is poorly understood. The present study investigated the phase II metabolism of feruloylquinic acids with selected human sulfotransferases (SULT1A1 and SULT1E1) and uridine 5'-diphosphoglucuronosyltransferases (UGT1A1 and UGT1A9). For unequivocal metabolite identification, the chemical synthesis of two potential human metabolites of 5-O-Feruloylquinic acid, the 4'-sulfated and 4'-O-glucuronidated conjugates, has been performed for the first time. Following incubation with human SULT1A1 or SULT1E1, formation of 5-O-Feruloylquinic acid 4'-O-sulfate was confirmed by matching its HPLC and MS data with those of the authentic standard. On the other hand, no glucuronide conjugates were detected after incubation with human uridine 5'-diphosphoglucuronosyltransferases. These results suggest that sulfation can take place on the ferulic acid moiety of feruloylquinic acids and may be a major metabolic pathway for feruloylquinic acids in humans.
Hydroxycinnamic acid derivatives occurring in Cichorium endivia vegetables.[Pubmed:18515031]
J Pharm Biomed Anal. 2008 Sep 29;48(2):472-6.
The hydroxycinnamic acid derivatives found in Chicorium endivia var. crispum and var. latifolium polyphenolic extracts were detected and characterized by high-performance liquid chromatography (HPLC) combined with photodiode array detector (DAD) and electrospray ionization-tandem mass spectrometry (ESI-MS/MS). The method provides data (molecular weight and diagnostic fragment ions) on the molecular structure of compounds. The combined approach enabled identification of four hydroxycinnamic derivatives in each chicory extract; three derivatives (5-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid, and 5-O-Feruloylquinic acid) were found in both chicories, while 3,5-di-O-caffeoylquinic acid was typical of var. crispum and cis-caftaric acid of var. latifolium.
A novel efficient and versatile route to the synthesis of 5-O-feruloylquinic acids.[Pubmed:18327321]
Org Biomol Chem. 2008 Mar 21;6(6):986-7.
A novel synthesis of 5-O-Feruloylquinic acid, a polyphenolic compound found in coffee beans, and its methyl ester derivative has been optimized. The sequence involves 6 steps and is compatible with the preparation of potential human metabolites of these compounds. The key reaction is a Knoevenagel condensation of 4-hydroxy-3-methoxy-benzaldehyde and a malonate ester of quinic acid.


