GlycitinCAS# 40246-10-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40246-10-4 | SDF | Download SDF |
| PubChem ID | 187808 | Appearance | White powder |
| Formula | C22H22O10 | M.Wt | 446.41 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Glycitein 7-O-β-glucoside | ||
| Solubility | DMSO : ≥ 38 mg/mL (85.13 mM) *"≥" means soluble, but saturation unknown. | ||
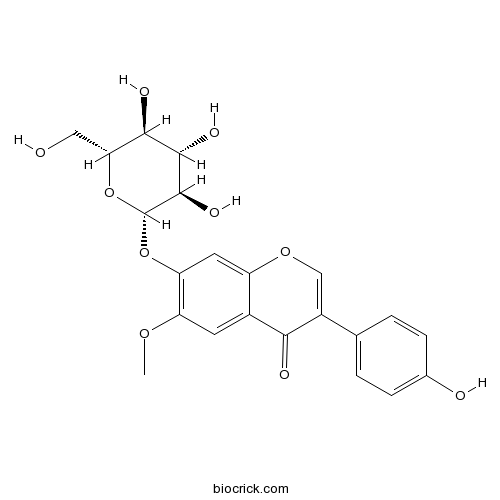
| Chemical Name | 3-(4-hydroxyphenyl)-6-methoxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | ||
| SMILES | COC1=C(C=C2C(=C1)C(=O)C(=CO2)C3=CC=C(C=C3)O)OC4C(C(C(C(O4)CO)O)O)O | ||
| Standard InChIKey | OZBAVEKZGSOMOJ-MIUGBVLSSA-N | ||
| Standard InChI | InChI=1S/C22H22O10/c1-29-15-6-12-14(30-9-13(18(12)25)10-2-4-11(24)5-3-10)7-16(15)31-22-21(28)20(27)19(26)17(8-23)32-22/h2-7,9,17,19-24,26-28H,8H2,1H3/t17-,19-,20+,21-,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glycitin has antibacterial, antiviral, anti-obese,anti-diabetic and estrogenic activities and may exert preventative effects on alcoholism, osteonecrosis,cardiovascular and cerebrovascular diseases and some types of cancer. It has a protective effect on skin aging by inhibiting of MMP-1 and increasing of collagen through ERK/JNK/P38 down-regulation, shows good inhibitory effect on α-glucosidase with IC50 of 0.5646 mg/mL. |
| Targets | MMP(e.g.TIMP) | ERK | JNK | p38MAPK |
| In vitro | Effects of genistein, daidzein and glycitein on the osteogenic and adipogenic differentiation of bone marrow stromal cells and on the adipogenic trans-differentiation of osteoblasts[Reference: WebLink]Acta Pharmacol. Sin., 2005, 26(9):1081-6.
|
| In vivo | Anti-obese and anti-diabetic effects of a mixture of daidzin and glycitin on C57BL/6J mice fed with a high-fat diet.[Pubmed: 25209298]Biosci Biotechnol Biochem. 2015 Jan;79(1):117-23.
The Protective Effect of Glycitin on UV-induced Skin Photoaging in Human Primary Dermal Fibroblast[Reference: WebLink]J. Korean Soc. App. Bi., 2014, 57(4):463-8.Exposure of strong and repeated UV on the skin leads to skin aging, characterized with wrinkling, sagging, dyspigmentation, and laxity. Numerous studies revealed that Matrix metalloproteinases are related to skin aging and functions as degrading enzyme of various types of collagen.
|
| Kinase Assay | Research on Structure Identification of Glycitin from Glycyrrhiza and Its Inhibitory Activity Against α-Glucosidase[Reference: WebLink]Characterization of a β-glucosidase from Sulfolobus solfataricus for isoflavone glycosides.[Pubmed: 21898127]Biotechnol Lett. 2012 Jan;34(1):125-9.The specific activity of a recombinant β-glucosidase from Sulfolobus solfataricus for isoflavones was: daidzin > Glycitin > genistin > malonyl genistin > malonyl daidzin > malonyl Glycitin. The hydrolytic activity of this enzyme for daidzin was highest at pH 5.5 and 90°C with a half-life of 18 h, a K (m) of 0.5 mM, and a k (cat) of 2532 s(-1). The enzyme converted 1 mM daidzin to 1 mM daidzein after 1 h with a molar yield of 100% and a productivity of 1 mM h(-1). Among β-glucosidases, that from S. solfataricus β had the highest thermostability, k (cat), k (cat)/K (m), conversion yield, and productivity in the hydrolysis of daidzin. Sci. Technol. Rev., 2014, 32(16):29-33.An isoflavone Glycitin is isolated from the licorice, which has some inhibitory effect on α-glucosidase. The compound has been isolated from Glycyrrhiza for the first time. The inhibition activity against α-glucosidase is tested in vitro, and this compound shows good inhibition effect on α-glucosidase with IC50of 0.5646 mg·mL- 1, better than the position control acarbose. The results of kinetic experiments show that the depressant effect of Glycitin is non competitive inhibition and the constant of Kmis 8.1571 mol·L-1. The value of Kiis 1.318 mg·mL-1 calculated by the Dixon equation. |
| Cell Research | Glycitin regulates osteoblasts through TGF-β or AKT signaling pathways in bone marrow stem cells.[Pubmed: 27882117 ]Exp Ther Med. 2016 Nov;12(5):3063-3067.Cell lines:Bone marrow stem cells (BMSCs) |
| Animal Research | Comparative study on reduction of bone loss and lipid metabolism abnormality in ovariectomized rats by soy isoflavones, daidzin, genistin, and glycitin.[Pubmed: 11305597]Biol Pharm Bull. 2001 Apr;24(4):368-72.Animal Models: Female Sprague-Dawley rats |

Glycitin Dilution Calculator

Glycitin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2401 mL | 11.2005 mL | 22.4009 mL | 44.8019 mL | 56.0023 mL |
| 5 mM | 0.448 mL | 2.2401 mL | 4.4802 mL | 8.9604 mL | 11.2005 mL |
| 10 mM | 0.224 mL | 1.12 mL | 2.2401 mL | 4.4802 mL | 5.6002 mL |
| 50 mM | 0.0448 mL | 0.224 mL | 0.448 mL | 0.896 mL | 1.12 mL |
| 100 mM | 0.0224 mL | 0.112 mL | 0.224 mL | 0.448 mL | 0.56 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Glycitin is a natural isoflavone isolated from legumes; promotes the proliferation of bone marrow stromal cells and osteoblasts and suppresses bone turnover.
References:
[1]. Uesugi T, et al. Comparative study on reduction of bone loss and lipid metabolism abnormality in ovariectomized rats by soy isoflavones, daidzin, genistin, and glycitin. Biol Pharm Bull. 2001 Apr;24(4):368-72.
[2]. Li XH, et al. Effect of daidzin, genistin, and glycitin on osteogenic and adipogenic differentiation of bone marrow stromal cells and adipocytic transdifferentiation of osteoblasts. Acta Pharmacol Sin. 2005 Sep;26(9):1081-6.
- 5-O-Feruloylquinic acid
Catalog No.:BCN3788
CAS No.:40242-06-6
- H-Hyp-OMe.HCl
Catalog No.:BCC3248
CAS No.:40216-83-9
- H-Orn-OMe.2HCl
Catalog No.:BCC3001
CAS No.:40216-82-8
- Fmoc-β-Homo-Gln(Trt)-OH
Catalog No.:BCC2647
CAS No.:401915-55-7
- Fmoc-β-homo-Arg(Pbf)-OH
Catalog No.:BCC2649
CAS No.:401915-53-5
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- SKA 31
Catalog No.:BCC7743
CAS No.:40172-65-4
- Boc-N-Me-Phe.DCHA
Catalog No.:BCC3348
CAS No.:40163-88-0
- Erucifoline
Catalog No.:BCN2081
CAS No.:40158-95-0
- H-Arg-pNA.2HCl
Catalog No.:BCC2858
CAS No.:40127-11-5
- Ceftaroline fosamil
Catalog No.:BCC5266
CAS No.:400827-46-5
- 20-Hydroxyganoderic acid G
Catalog No.:BCN8231
CAS No.:400604-12-8
- ONO-AE3-208
Catalog No.:BCC1822
CAS No.:402473-54-5
- Acetylcimigenol 3-O-alpha-L-arabinopyranside
Catalog No.:BCN1447
CAS No.:402513-88-6
- DMeOB
Catalog No.:BCC7213
CAS No.:40252-74-2
- NSC 693868
Catalog No.:BCC7208
CAS No.:40254-90-8
- Firategrast
Catalog No.:BCC1575
CAS No.:402567-16-2
- Deacetylcinobufagin
Catalog No.:BCN2720
CAS No.:4026-95-3
- SB 399885 hydrochloride
Catalog No.:BCC7595
CAS No.:402713-81-9
- 7-Methoxyneochamaejasmine A
Catalog No.:BCN3134
CAS No.:402828-38-0
- DR 4485 hydrochloride
Catalog No.:BCC7990
CAS No.:402942-53-4
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- MDL 12330A hydrochloride
Catalog No.:BCC7066
CAS No.:40297-09-4
- Cassyfiline
Catalog No.:BCN4763
CAS No.:4030-51-7
Characterization of a beta-glucosidase from Sulfolobus solfataricus for isoflavone glycosides.[Pubmed:21898127]
Biotechnol Lett. 2012 Jan;34(1):125-9.
The specific activity of a recombinant beta-glucosidase from Sulfolobus solfataricus for isoflavones was: daidzin > Glycitin > genistin > malonyl genistin > malonyl daidzin > malonyl Glycitin. The hydrolytic activity of this enzyme for daidzin was highest at pH 5.5 and 90 degrees C with a half-life of 18 h, a K (m) of 0.5 mM, and a k (cat) of 2532 s(-1). The enzyme converted 1 mM daidzin to 1 mM daidzein after 1 h with a molar yield of 100% and a productivity of 1 mM h(-1). Among beta-glucosidases, that from S. solfataricus beta had the highest thermostability, k (cat), k (cat)/K (m), conversion yield, and productivity in the hydrolysis of daidzin.
Anti-obese and anti-diabetic effects of a mixture of daidzin and glycitin on C57BL/6J mice fed with a high-fat diet.[Pubmed:25209298]
Biosci Biotechnol Biochem. 2015;79(1):117-23.
We investigated the effects of a mixture of daidzin and Glycitin, which are the glycoside-form isoflavones of daidzein and glycitein, respectively, on body weight, lipid levels, diabetic markers, and metabolism in a high-fat diet (HF) fed C57BL/6J mice for 92 days. The mice were divided into basic diet group (CON), HF group, and HF companied with the isoflavone mixture group (HFISO). Results showed that mice in HFISO had a significantly lower body weight and adipose tissue compared to HF group. Blood glucose, serum HbA1c, and serum insulin also showed lower levels in HFISO group. In addition, higher hepatic GSH level and lower serum 8-hydroxy-2'-deoxyguanosine (8-OHdG) level were found in HFISO group mice. This suggests that the regulation of oxidative stress by daidzin and Glycitin was closely related to the suppression of adipose tissue and the progression of diabetes.
Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39.[Pubmed:21942422]
J Agric Food Chem. 2011 Nov 9;59(21):11764-71.
The aim of this study was to identify the bitter receptor(s) that recognize the bitter taste of the soy isoflavone genistein. Screening of all 25 human bitter receptors revealed genistein as agonist of hTAS2R14 and hTAS2R39. Genistein displayed threshold values of 4 and 8 muM on hTAS2R14 and hTAS2R39 and EC(50) values of 29 and 49 muM, respectively. In addition, the behavior of structurally similar isoflavonoids was investigated. Although the two receptors are not closely related, the results for hTAS2R14 and hTAS2R39 were similar toward most isoflavonoid aglycones. By trend, threshold values were slightly lower on hTAS2R14. Glucosylation of isoflavones seemed to inhibit activation of hTAS2R14, whereas four of five glucosylated isoflavones were agonists of hTAS2R39, namely, Glycitin, genistin, acetylgenistin, and malonylgenistin. A total of three hydroxyl substitutions of the A- and B-rings of the isoflavonoids seemed to be more favorable for receptor activation than fewer hydroxyl groups. The concentration of the trihydroxylated genistein in several soy foods exceeds the determined bitter receptor threshold values, whereas those of other soy isoflavones are around or below their respective threshold value. Despite its low concentration, genistein might be one of the main contributors to the bitterness of soy products. Furthermore, the bioactive isoflavonoids equol and coumestrol activated both receptors, indicating that their sensory impact should be considered when used as food ingredients.


