Clasto-Lactacystin β-lactoneProteasome inhibitor CAS# 154226-60-5 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154226-60-5 | SDF | Download SDF |
| PubChem ID | 9794358 | Appearance | Powder |
| Formula | C10H15NO4 | M.Wt | 213.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 197.79 mM in DMSO | ||
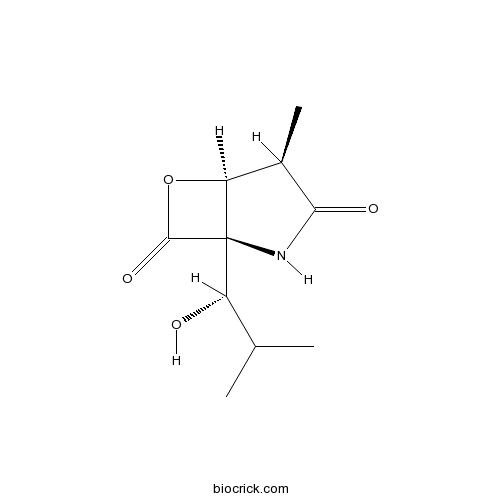
| Chemical Name | (1R,4R,5S)-1-[(1S)-1-hydroxy-2-methylpropyl]-4-methyl-6-oxa-2-azabicyclo[3.2.0]heptane-3,7-dione | ||
| SMILES | CC1C2C(C(=O)O2)(NC1=O)C(C(C)C)O | ||
| Standard InChIKey | FWPWHHUJACGNMZ-NBBQQVJHSA-N | ||
| Standard InChI | InChI=1S/C10H15NO4/c1-4(2)6(12)10-7(15-9(10)14)5(3)8(13)11-10/h4-7,12H,1-3H3,(H,11,13)/t5-,6+,7+,10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Clasto-Lactacystin β-lactone Dilution Calculator

Clasto-Lactacystin β-lactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6898 mL | 23.4489 mL | 46.8977 mL | 93.7954 mL | 117.2443 mL |
| 5 mM | 0.938 mL | 4.6898 mL | 9.3795 mL | 18.7591 mL | 23.4489 mL |
| 10 mM | 0.469 mL | 2.3449 mL | 4.6898 mL | 9.3795 mL | 11.7244 mL |
| 50 mM | 0.0938 mL | 0.469 mL | 0.938 mL | 1.8759 mL | 2.3449 mL |
| 100 mM | 0.0469 mL | 0.2345 mL | 0.469 mL | 0.938 mL | 1.1724 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cell-permeable. A highly specific, potent and irreversible proteasome inhibitor. Lactacystin acts as a precursor for clasto-lactacystin β-lactone and the latter compound is at least 10 times more active than the parent Lactacystin
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Berberrubine
Catalog No.:BCN2651
CAS No.:15401-69-1
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
Enantioselective total syntheses of (-)-clasto-lactacystin beta-lactone and 7-epi-(-)-clasto-lactacystin beta-lactone.[Pubmed:16391758]
Org Biomol Chem. 2006 Jan 21;4(2):193-5.
An alkylidene carbene 1,5-CH insertion has been used as a key step in an efficient enantioselective total synthesis of (-)-clasto-lactacystin beta-lactone, and its C7-epimer. An additional noteworthy feature of the synthesis is the use of a novel oxidative deprotection procedure, utilizing DMDO, for the conversion of a late-stage benzylidene acetal into a primary alcohol and a secondary benzoate ester.
Prediction of the mechanism of action of omuralide (clasto-lactacystin beta-lactone) on human cathepsin A based on a structural model of the yeast proteasome beta5/PRE2-subunit/omuralide complex.[Pubmed:16870514]
Biochim Biophys Acta. 2006 Aug;1764(8):1372-80.
Cathepsin A (CathA) is a lysosomal serine carboxypeptidase that exhibits homology and structural similarity to the yeast and wheat serine carboxypeptidases (CPY and CPW) belonging to the alpha/beta-hydrolase fold family. Human CathA (hCathA) and CPW have been demonstrated to be inhibited by a proteasome (threonine protease) inhibitor, lactacystin, and its active derivative, omuralide (clasto-lactacystin beta-lactone), as well as chymostatin. A hCathA/omuralide complex model constructed on the basis of the X-ray crystal structures of the CPW/chymostatin complex and the yeast proteasome beta-subunit (beta5/PRE2)/omuralide one predicted that the conformation of omuralide in the active-site cleft of proteasome beta5/PRE2 should be very similar to that of chymostatin at the S1 catalytic subsites in the hCathA- and CPW-complexes. The relative positions of the glycine residues, i.e., Gly57 in hCathA, Gly53 in CPW, and Gly47 in beta5/PRE2, present in the oxyanion hole of each enzyme were also highly conserved. These results suggest that omuralide might inhibit hCathA and CPW at the S1 subsite in their active-site clefts through direct binding to the active serine residue.
Effect of proteasome inhibitor clasto-lactacystin-beta-lactone on the proteome of the haloarchaeon Haloferax volcanii.[Pubmed:17600071]
Microbiology. 2007 Jul;153(Pt 7):2271-80.
Proteasomes play key roles in a variety of eukaryotic cell functions, including translation, transcription, metabolism, DNA repair and cell-cycle control. The biological functions of these multicatalytic proteases in archaea, however, are poorly understood. In this study, Haloferax volcanii was used as a model to determine the influence the proteasome-specific inhibitor clasto-lactacystin-beta-lactone (cLbetaL) has on archaeal proteome composition. Addition of 20-30 microM cLbetaL had a widespread effect on the proteome, with a 38-42 % increase in the number of 2-D gel electrophoresis (2-DE) protein spots, from an average of 627 to 1036 spots. Protein identities for 17 of the spots that were easily separated by 2-DE and unique and/or increased 2- to 14-fold in the cLbetaL-treated cells were determined by tandem mass spectrometry (MS/MS). These included protein homologues of the DJ-1/ThiJ family, mobilization of sulfur system, translation elongation factor EF-1 A, ribosomal proteins, tubulin-like FtsZ, divalent metal ABC transporter, dihydroxyacetone kinase DhaL, aldehyde dehydrogenase and 2-oxoacid decarboxylase E1beta. Based on these results, inhibition of H. volcanii proteasomes had a global influence on proteome composition, including proteins involved in central functions of the cell.
Utility of the ammonia-free Birch reduction of electron-deficient pyrroles: total synthesis of the 20s proteasome inhibitor, clasto-lactacystin beta-lactone.[Pubmed:15864801]
Chemistry. 2005 Jul 4;11(14):4227-38.
A new synthesis of the 20S proteasome inhibitor clasto-lactacystin beta-lactone is described. Our route to this important natural product involves the partial reduction of an electron deficient pyrrole as a key step. By judicious choice of enolate counterion, we were able to exert complete control over the stereoselectivity of the reduction/aldol reaction. Early attempts to complete the synthesis by using a C-4 methyl substituted pyrrole are described in full, together with our attempts to promote regioselective elimination of a tertiary alcohol. The lessons learnt from this first approach led us to develop another, and ultimately successful, route that introduced the C-4 methyl group at a late stage in the synthesis. Our successful route is then described and this contains several highly stereoselective steps including a cis-dihydroxylation and an enolate methylation. The final synthesis proceeds in just 13 steps and in 15 % overall yield making it an extremely efficient route to this valuable compound.


