IsatidineCAS# 15503-86-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

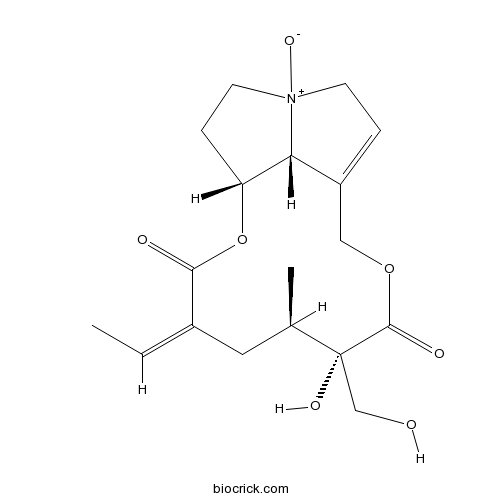
| Cas No. | 15503-86-3 | SDF | Download SDF |
| PubChem ID | 5281734 | Appearance | White powder |
| Formula | C18H25NO7 | M.Wt | 367.40 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Retrorsine N-oxide | ||
| Solubility | Soluble in methanol; sparingly soluble in water | ||
| SMILES | CC=C1CC(C(C(=O)OCC2=CC[N+]3(C2C(CC3)OC1=O)[O-])(CO)O)C | ||
| Standard InChIKey | IDIMIWQPUHURPV-WTWIWYCDSA-N | ||
| Standard InChI | InChI=1S/C18H25NO7/c1-3-12-8-11(2)18(23,10-20)17(22)25-9-13-4-6-19(24)7-5-14(15(13)19)26-16(12)21/h3-4,11,14-15,20,23H,5-10H2,1-2H3/b12-3-/t11-,14-,15-,18-,19?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isatidine shows in vitro genotoxicity in Hep G2/SCGE assays. 2. Isatidine and sceleratine slightly raise blood pressure in cats following intravenous injection, and stimulate isolated guinea pigs' uteri. |

Isatidine Dilution Calculator

Isatidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7218 mL | 13.6091 mL | 27.2183 mL | 54.4366 mL | 68.0457 mL |
| 5 mM | 0.5444 mL | 2.7218 mL | 5.4437 mL | 10.8873 mL | 13.6091 mL |
| 10 mM | 0.2722 mL | 1.3609 mL | 2.7218 mL | 5.4437 mL | 6.8046 mL |
| 50 mM | 0.0544 mL | 0.2722 mL | 0.5444 mL | 1.0887 mL | 1.3609 mL |
| 100 mM | 0.0272 mL | 0.1361 mL | 0.2722 mL | 0.5444 mL | 0.6805 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pancuronium dibromide
Catalog No.:BCC4578
CAS No.:15500-66-0
- Ac-Arg-OH.2H2O
Catalog No.:BCC2855
CAS No.:155-84-0
- Rhaponiticin
Catalog No.:BCN5392
CAS No.:155-58-8
- Methscopolamine
Catalog No.:BCC4577
CAS No.:155-41-9
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
- Erysenegalensein E
Catalog No.:BCN3979
CAS No.:154992-17-3
- BAY-u 9773
Catalog No.:BCC7576
CAS No.:154978-38-8
- LL 37
Catalog No.:BCC8027
CAS No.:154947-66-7
- Kaempferol 3,7,4'-trimethylether
Catalog No.:BCN4087
CAS No.:15486-34-7
- 3,5-Dihydroxy-4',7-dimethoxyflavone
Catalog No.:BCN1691
CAS No.:15486-33-6
- Eleutheroside C
Catalog No.:BCN1690
CAS No.:15486-24-5
- 4-Acetoxycinnamic acid
Catalog No.:BCN5026
CAS No.:15486-19-8
- Usaramine
Catalog No.:BCN2121
CAS No.:15503-87-4
- 4-Hydroxy-3-(3-methyl-2-butenoyl)-5-(3-methyl-2-butenyl)benzoic acid
Catalog No.:BCN1554
CAS No.:155051-85-7
- N-[2-(Piperidinylamino)ethyl]-4-iodobenzamide
Catalog No.:BCC6784
CAS No.:155054-42-5
- SM-21 maleate
Catalog No.:BCC6780
CAS No.:155059-42-0
- 24,25-Dihydroxycycloartan-3-one
Catalog No.:BCN1692
CAS No.:155060-48-3
- 11-Deoxyalisol B
Catalog No.:BCN3359
CAS No.:155073-73-7
- 6-ethyl-3-methyl-4-oxo-4H-pyran-2-carboxylic acid
Catalog No.:BCC8270
CAS No.:1551-49-1
- 2-[1-(4-Piperonyl)piperazinyl]benzothiazole
Catalog No.:BCC6771
CAS No.:155106-73-3
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- Plerixafor octahydrobromide
Catalog No.:BCC9123
CAS No.:155148-32-6
- Physapruin A
Catalog No.:BCN7576
CAS No.:155178-03-3
Micronucleus formation in human lymphocytes and in the metabolically competent human hepatoma cell line Hep-G2: results with 15 naturally occurring substances.[Pubmed:11299780]
Anticancer Res. 2001 Jan-Feb;21(1A):461-9.
To examine the concordance of two metabolizing systems for use in genotoxocity testing with the micronucleus test, 15 naturally occurring substances (arecoline, the plant extract aristolochic acid, beta-asarone, benzyl acetate, coumarin, emodine, Isatidine dihydrate, monocrotaline, psoralen, reserpine, retrorsine, safrole, sanguinarine chloride, tannin and thiourea) were tested for their genotoxicity in the cytokinesis-block micronucleus test in vitro with human lymphocytes and in the presence and the absence of an exogenous metabolizing system from rat liver S9-mix and the metabolically competent human hepatoma cell line Hep-G2. Arecoline, the plant extract aristolochic acid, psoralen and tannin caused a significant increase in the number of micronuclei in human lymphocytes in the presence and the absence of an exogenous metabolising system from rat liver S9-mix and the metabolically competent human hepatoma cell line Hep-G2. A significant increase in the number of micronuclei with beta-asarone, coumarin, monocrotaline and retrorsine could be detected in the presence of S9-mix and the cell line Hep-G2. Benzyl acetate, emodine, Isatidine dihydrate, reserpine, safrole, sanguinarine chloride and thiourea did not reveal any micronucleus inducing activity in either human lymphocytes or in Hep-G2. In addition to the other Hep-G2 results in the literature, this human hepatoma cell line could have a useful potential in the in vitro micronucleus test.
Evaluation of the single cell gel electrophoresis assay with human hepatoma (Hep G2) cells.[Pubmed:10882898]
Mutat Res. 2000 Jul 10;468(2):213-25.
Human Hep G2 cells have retained the activities of phase I and phase II enzymes which are involved in the metabolism of environmental genotoxins. The present study describes the results of single cell gel electrophoresis (SCGE) assays with a panel of different model compounds with these cells. With genotoxic carcinogens such as aflatoxin B(1) (AFB(1)), benzo(a)pyrene (B(a)P), nitrosodimethylamine (NDMA) and cyclophosphamide (CP), statistically significant dose dependent induction of DNA migration was measured. With the two heterocyclic amines, 2-amino-3-methyl-3H-imidazo[4, 5-f]quinoline (IQ) and 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1), and also with rodent carcinogens such as safrole, hexamethylphosphoramide (HMPA) and the pyrrolizidine alkaloid Isatidine, which give negative results in other in vitro genotoxicity tests, positive results were obtained in Hep G2/SCGE assays. Nitrosomethylurea (NMU) was the only directly acting compound tested in the study and was by far (ca. 10(3)-fold) more active than the corresponding nitrosamine. The exposure concentrations required to cause significant effects varied over a broad range. The most pronounced effect was seen with AFB(1) (0.008 microM) followed by HMPA (15 microM), B(a)P (25 microM), NMU (100 microM), isatidin (500 microM), CP (900 microM), IQ (1200 microM), safrol (4000 microM), and NDMA (90x10(3) microM). Numbers in parenthesis give the lowest concentrations, which caused a significant increase of DNA migration. With two compounds, namely, the non-carcinogen pyrene and the synthetic hormone tamoxifen (TF), negative results were obtained under all test conditions. These findings are in agreement with the results of recent investigations which indicated that human hepatocytes are unable to convert TF to DNA reactive metabolites, whereas it is activated by rat liver cells and causes DNA adducts in these cells. Comparisons of the present results with data from earlier experiments indicate that the Hep G2/SCGE assay enables the detection of genotoxins including compounds which give misleading results in other in vitro genotoxicity tests and appears to be an alternative to tests with primary liver cells from laboratory animals.
The clastogenic potential in vitro of pyrrolizidine alkaloids employing hepatocyte metabolism.[Pubmed:1378549]
Mutat Res. 1992 Jul;282(3):169-76.
Three pyrrolizidine alkaloids (PAs), monocrotaline, retrorsine and Isatidine, were tested for their clastogenic activity under different conditions of metabolic activation in vitro. All three compounds exhibited a weak activity when V79 cells were treated at very high concentrations for 18 h in the absence of a metabolizing system. Short-term (2 h) treatment with rat liver S9 mix led to a strong and concentration-dependent increase in chromosomal aberrations for retrorsine. Isatidine was not mutagenic with S9 mix and monocrotaline was positive at high concentrations only. In contrast, a prolonged treatment (18 h) in vitro under activation conditions in the presence of primary hepatocytes led to clear concentration-dependent positive responses for all three PAs investigated. Particularly the results with Isatidine demonstrate that in vitro tests using S9 mix for metabolization can generate misleading results. It is not clear whether the results could be attributed to a better activation of the test compounds by intact hepatocytes in comparison to S9 mix or if the fact that only hepatocytes allow a treatment for the whole culture period under activation conditions was more important. Owing to its strong cytotoxicity the exposure to S9 mix is generally limited to 2-4 h, limiting also the exposure of the target cells to a test chemical as well as its metabolites. The results presented show significant differences in mutagenic potency of PAs due to variations in the activation system. This underlines the usefulness of primary hepatocytes, e.g., for the detection of pre-mutagens. The PAs investigated are present in plants which are used for phytotherapeutic medicinal products. They do not contribute to their efficacy and are, therefore, not to be tolerated in amounts that may impose a risk for the user.


