LCL161Antagonist of IAPs inhibitor CAS# 1005342-46-0 |
- BV6
Catalog No.:BCC5372
CAS No.:1001600-56-1
- Birinapant (TL32711)
Catalog No.:BCC2250
CAS No.:1260251-31-7
- UC 112
Catalog No.:BCC8042
CAS No.:383392-66-3
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- GDC-0152
Catalog No.:BCC2252
CAS No.:873652-48-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1005342-46-0 | SDF | Download SDF |
| PubChem ID | 24737642 | Appearance | Powder |
| Formula | C26H33FN4O3S | M.Wt | 500.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (199.75 mM; Need ultrasonic) | ||
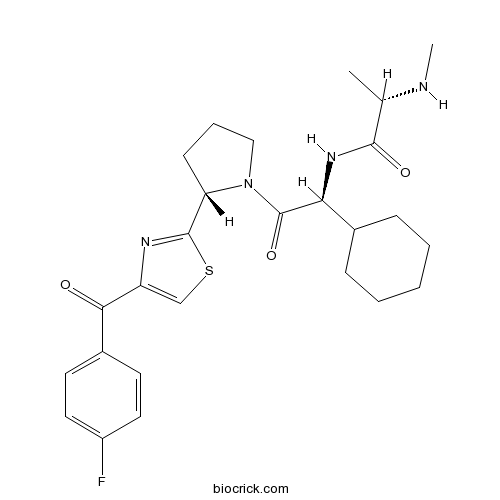
| Chemical Name | (2S)-N-[(1S)-1-cyclohexyl-2-[(2S)-2-[4-(4-fluorobenzoyl)-1,3-thiazol-2-yl]pyrrolidin-1-yl]-2-oxoethyl]-2-(methylamino)propanamide | ||
| SMILES | CC(C(=O)NC(C1CCCCC1)C(=O)N2CCCC2C3=NC(=CS3)C(=O)C4=CC=C(C=C4)F)NC | ||
| Standard InChIKey | UFPFGVNKHCLJJO-SSKFGXFMSA-N | ||
| Standard InChI | InChI=1S/C26H33FN4O3S/c1-16(28-2)24(33)30-22(17-7-4-3-5-8-17)26(34)31-14-6-9-21(31)25-29-20(15-35-25)23(32)18-10-12-19(27)13-11-18/h10-13,15-17,21-22,28H,3-9,14H2,1-2H3,(H,30,33)/t16-,21-,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LCL161 is a novel IAP inhibitor, inhibits XIAP activity in HEK293 cell with IC50 of 35 nM, also inhibits cIAP1 activity in MDA-MB-231 cell with IC50 of 0.4 nM.In Vitro:LCL161 shows anti-proliferative effects and reduces cell viability significantly in Hep3B (IC50=10.23 μM) and PLC5 (IC50=19.19 μM) cells in a dose-dependent manner. LCL161 induces apoptosis significantly in both the sensitive cell lines in a dose-dependent manner. LCL161 significantly down regulates the expression of cIAP1, starting at very low concentrations. LCL161 at low concentrations inhibits cIAP1 starting at the concentration of 0.5 nM[2]. LCL161 is a small molecule oral IAP antagonist in development for use in combination with cytotoxic agents. The effect of LCL161 on CYP3A4/5 (CYP3A) activity is investigated in vitro. Results in human liver microsomes indicated LCL161 inhibited CYP3A in a concentration- and time-dependent manner (KI of 0.797 µM and Kinact of 0.0803 min-1). LCL161 activates human PXR in a reporter gene assay and induced CYP3A4 mRNA up to ~5-fold in human hepatocytes[3].In Vivo:Tumor-bearing mice are treated with vehicle or LCL161 p.o. at a dose of 50 mg/kg/day, or SC-2001 p.o. at a dose of 10 mg/kg/day, 5 days a week, or in combination for the duration of the study. Tumor growth is significantly inhibited by co-treatment with SC2001 and LCL161 and tumor size in the co-treatment group is only one third of that of the control group at the end of the study[2]. LCL161 is a first-in-class oral Smac mimetic shown to induce degradation of cIAP1 and cleavage of caspase 3 in mouse xenograft models[4]. References: | |||||

LCL161 Dilution Calculator

LCL161 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9975 mL | 9.9874 mL | 19.9748 mL | 39.9497 mL | 49.9371 mL |
| 5 mM | 0.3995 mL | 1.9975 mL | 3.995 mL | 7.9899 mL | 9.9874 mL |
| 10 mM | 0.1997 mL | 0.9987 mL | 1.9975 mL | 3.995 mL | 4.9937 mL |
| 50 mM | 0.0399 mL | 0.1997 mL | 0.3995 mL | 0.799 mL | 0.9987 mL |
| 100 mM | 0.02 mL | 0.0999 mL | 0.1997 mL | 0.3995 mL | 0.4994 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LCL161 is a small molecular antagonist of the inhibitor of apoptosis (IAP) with IC50 value of 10.23 μM in Hep3B cells [1].
IAP is a family of proteins which are firstly found in virus and to inhibit the apoptosis of the infected hosts. It contains eight proteins in human. Since they were always found to overexpress in variety of malignant tumors, the IAP are thought to be appropriate targets in cancer therapy. SMAC (second mitochondria-derived activator of caspases) is the first-identified antagonist of IAP. It binds to XIAP within the BIR2/3 domain through its N-terminal segment. As a mimetic of SMAC, LCL161 is designed to be the inhibitor of both XIAP and cIAP1/2 [1].
LCL161 showed significant inhibition of cell proliferation and viability in two human hepatocellular carcinoma (HCC) cells, Hep3B and PLC5. The IC50 values were 10 and 19 μM, respectively. However, LCL161 had no effect in the two other HCC cell lines, Sk-Hep1 (IC50 value of 224 μM) and Huh-7 (IC50 value of 228 μM). The difference of the effects is found to dependent on the expression of Bcl-2 in cells. For the ALL cells, LCL161 exerted growth inhibition with IC50 values of 9.3 and 0.25 μM, respectively. LCL161 also showed effect on the ALCL cell line Karpas-299 with IC50 value of 1.6 μM [1, 2].
In vivo, LCL161 markedly affected the distribution of EFS in many solid tumor xenograft models. It also caused growth delay in some tumors such as osteosarcoma, neuroblastoma and glioblastoma at dose of 30 mg/kg orally. Besides that, LCL161 administration caused significant growth inhibition in EW-5 and BT-39 glioblastoma but not in BT-28. Moreover, the combination therapy of LCL161 and the adeno-associated virus bacteriophage-tumor necrosis factor-α (AAVP-TNF-α) has been reported to has synergistic anti-tumor effects and delayed treatment resistance in mice models of tumor xenografts [1, 2 and 3].
References:
1.Chen K F, Lin J P, Shiau C W, et al. Inhibition of Bcl-2 improves effect of LCL161, a SMAC mimetic, in hepatocellular carcinoma cells. Biochemical pharmacology, 2012, 84(3): 268-277.
2.Houghton P J, Kang M H, Reynolds C P, et al. Initial testing (stage 1) of LCL161, a SMAC mimetic, by the Pediatric Preclinical Testing Program. Pediatric blood & cancer, 2012, 58(4): 636-639.
3.Yuan Z, Syrkin G, Adem A, et al. Blockade of inhibitors of apoptosis (IAPs) in combination with tumor-targeted delivery of tumor necrosis factor-α leads to synergistic antitumor activity. Cancer gene therapy, 2012, 20(1): 46-56.
- CVT 10216
Catalog No.:BCC5606
CAS No.:1005334-57-5
- Gelomulide N
Catalog No.:BCN6641
CAS No.:1005212-02-1
- Aeruginolactone
Catalog No.:BCN3695
CAS No.:1005208-88-7
- TCS 2002
Catalog No.:BCC6074
CAS No.:1005201-24-0
- Blasticidin A
Catalog No.:BCN1830
CAS No.:100513-53-9
- Sterigmatocystin
Catalog No.:BCN6885
CAS No.:10048-13-2
- Gastrin I (human)
Catalog No.:BCC5958
CAS No.:10047-33-3
- Rosiridin
Catalog No.:BCN5970
CAS No.:100462-37-1
- FFN 511
Catalog No.:BCC7799
CAS No.:1004548-96-2
- 1-EBIO
Catalog No.:BCC6904
CAS No.:10045-45-1
- Dihydroresveratrol 3-O-glucoside
Catalog No.:BCN5821
CAS No.:100432-87-9
- Cobicistat (GS-9350)
Catalog No.:BCC2271
CAS No.:1004316-88-4
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- Monomethylsulochrin
Catalog No.:BCN7255
CAS No.:10056-14-1
- TC ASK 10
Catalog No.:BCC6301
CAS No.:1005775-56-3
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- NF 546
Catalog No.:BCC7804
CAS No.:1006028-37-0
- MK-2894
Catalog No.:BCC1757
CAS No.:1006036-87-8
- MK-2894 sodium salt
Catalog No.:BCC1758
CAS No.:1006036-88-9
- (-)-Epipinoresinol
Catalog No.:BCN3377
CAS No.:10061-38-8
- Desloratadine
Catalog No.:BCC4540
CAS No.:100643-71-8
- Ganoderenic acid A
Catalog No.:BCN3208
CAS No.:100665-40-5
- Ganoderenic acid B
Catalog No.:BCN7966
CAS No.:100665-41-6
- Ganoderenic acid C
Catalog No.:BCN3210
CAS No.:100665-42-7
[Effects of LCL161, a Smac mimetic on the proliferation and apoptosis in hepatocellular carcinoma cells].[Pubmed:27640787]
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016 Sep 28;41(9):898-904.
OBJECTIVE: To investigate the effects of LCL161, a Smac mimetic, on the proliferation and apoptosis in hepatocellular carcinoma cells and the underlying mechanisms. METHODS: The effect of LCL161 on the cell viability of HepG2 and SMMC7721 cells was measured by MTT assay. The effect of LCL161 at lower concentrations on the proliferation in hepatocellular carcinoma (HCC) cells was detected by colony formation assay. Apoptosis was assessed by flow cytometry with PI staining. The mitochondrial membrane potential was measured by JC-1 staining. The expression of PARP, p-Akt, cIAP1 and XIAP protein was analyzed by Western blot. RESULTS: LCL161 displayed notable antiproliferative activity on HCC cells at the concentrations of 1-16 mumol/L (P<0.01), with IC50 values of 4.3 and 4.9 mumol/L for HepG2 and SMMC7721 cells, respectively, after treatment for 48 h. LCL161 at lower concentrations obviously inhibited the colony formation of HCC cells. LCL161 induced significant apoptosis in HCC cells (P<0.01), and resulted in the apoptotic rate at (1.5+/-0.8)% or (1.8+/-0.6)% , (15.2+/-2.8)% or (12.2+/-2.4)%, (28.7+/-3.0)% or (22.4+/-2.7)%, (34.6+/-2.3)% or (30.2+/-2.4)% for HepG2 cells or SMMC7721 cells at the concentration of 0, 2, 4 or 8 mumol/L, respectively. The result of JC-1 staining indicated that the mitochondrial membrane potential of HCC cells was reduced by LCL161. In addition, LCL161 promoted the cleavage of PARP, down-regulated the protein expression of p-Akt, and degraded cIAP1. CONCLUSION: LCL161 possesses significant anti-proliferative activity and pro-apoptotic action in HepG2 and SMMC7721 cells, which might be correlated with reduction in mitochondrial membrane potential, down-regulation of p-Akt and degradation of cIAP1.
LCL161 increases paclitaxel-induced apoptosis by degrading cIAP1 and cIAP2 in NSCLC.[Pubmed:27737687]
J Exp Clin Cancer Res. 2016 Sep 30;35(1):158.
BACKGROUND: LCL161, a novel Smac mimetic, is known to have anti-tumor activity and improve chemosensitivity in various cancers. However, the function and mechanisms of the combination of LCL161 and paclitaxel in non-small cell lung cancer (NSCLC) remain unknown. METHODS: Cellular inhibitor of apoptotic protein 1 and 2 (cIAP1&2) expression in NSCLC tissues and adjacent non-tumor tissues were assessed by immunohistochemistry. The correlations between cIAP1&2 expression and clinicopathological characteristics, prognosis were analyzed. Cell viability and apoptosis were measured by MTT assays and Flow cytometry. Western blot and co-immunoprecipitation assay were performed to measure the protein expression and interaction in NF-kB pathway. siRNA-mediated gene silencing and caspases activity assays were applied to demonstrate the role and mechanisms of cIAP1&2 and RIP1 in lung cancer cell apoptosis. Mouse xenograft NSCLC models were used in vivo to determine the therapeutic efficacy of LCL161 alone or in combination with paclitaxel. RESULTS: The expression of cIAP1 and cIAP2 in Non-small cell lung cancer (NSCLC) tumors was significantly higher than that in adjacent normal tissues. cIAP1 was highly expressed in patients with late TNM stage NSCLC and a poor prognosis. Positivity for both cIAP1 and cIAP2 was an independent prognostic factor that indicated a poorer prognosis in NSCLC patients. LCL161, an IAP inhibitor, cooperated with paclitaxel to reduce cell viability and induce apoptosis in NSCLC cells. Molecular studies revealed that paclitaxel increased TNFalpha expression, thereby leading to the recruitment of various factors and the formation of the TRADD-TRAF2-RIP1-cIAP complex. LCL161 degraded cIAP1&2 and released RIP1 from the complex. Subsequently, RIP1 was stabilized and bound to caspase-8 and FADD, thereby forming the caspase-8/RIP1/FADD complex, which activated caspase-8, caspase-3 and ultimately lead to apoptosis. In nude mouse xenograft experiments, the combination of LCL161 and paclitaxel degraded cIAP1,2, activated caspase-3 and inhibited tumor growth with few toxic effects. CONCLUSION: Thus, LCL161 could be a useful agent for the treatment of NSCLC in combination with paclitaxel.
Smac mimetic LCL161 supports neuroblastoma chemotherapy in a drug class-dependent manner and synergistically interacts with ALK inhibitor TAE684 in cells with ALK mutation F1174L.[Pubmed:27655666]
Oncotarget. 2016 Nov 8;7(45):72634-72653.
Neuroblastoma is the most common extracranial solid tumor during infancy and childhood.Outcome of high-risk and late-stage disease remains poor despite intensive treatment regimens.Suppressing inhibitor of apoptosis proteins (IAPs) using Smac mimetics (SM) significantly sensitizes neuroblastoma (NB) cells for chemotherapy, however strongly dependent on the cytotoxic drug combined with SM.Therefore, a systematic analysis of the impact of SM in combination with different classes of chemotherapeutics was of crucial importance. Treatment of NB cell lines with SM LCL161 and vinca alkaloids revealed a strong synergistic inhibition of proliferation and significant induction of apoptosis in virtually all established and de novo NB cell lines (n=8).In contrast, combination of anthracyclines or topoisomerase inhibitors with LCL161 showed a synergism for single drugs and/or cell lines only.Furthermore, we could show that insensibility to LCL161-mediated sensitization for chemotherapeutics is associated with aberrant activation of anaplastic lymphoma kinase (ALK) by common mutation F1174L. Inhibition of ALK using TAE684 is able to overcome this resistance in a synergistic fashion, a finding that could be highly relevant for improvement of neuroblastoma therapy.
Smac mimetic LCL161 overcomes protective ER stress induced by obatoclax, synergistically causing cell death in multiple myeloma.[Pubmed:27494845]
Oncotarget. 2016 Aug 30;7(35):56253-56265.
Bcl2 and IAP families are anti-apoptotic proteins deregulated in multiple myeloma (MM) cells. Pharmacological inhibition of each of these families has shown significant activity only in subgroups of MM patients. Here, we have examined a broad-spectrum Bcl2 family inhibitor Obatoclax (OBX) in combination with a Smac mimetic LCL161 in MM cell lines and patient cells. LCL161/OBX combination induced synergistic cytotoxicity and anti-proliferative effects on a broad range of human MM cell lines. The cytotoxicity was mediated through inhibition of the IAPs, activation of caspases and up regulation of the pro-apoptotic proteins Bid, Bim, Puma and Noxa by the drug combination. In addition, we observed that OBX caused ER stress and activated the Unfolded Protein Response (UPR) leading to drug resistance. LCL161, however inhibited spliced Xbp-1, a pro-survival factor. In addition, we observed that OBX increased GRP78 localization to the cell surface, which then induced PI3K dependent Akt activation and resistance to cell death. LCL161 was able to block OBX induced Akt activation contributing to synergistic cell death. Our results support clinical evaluation of this combination strategy in relapsed refractory MM patients.


