TirasemtivFast-skeletal-troponin activator CAS# 1005491-05-3 |
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- TPT-260 Dihydrochloride
Catalog No.:BCC5172
CAS No.:2076-91-7
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1005491-05-3 | SDF | Download SDF |
| PubChem ID | 23729157 | Appearance | Powder |
| Formula | C12H14N4O | M.Wt | 230.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CK-2017357 | ||
| Solubility | DMSO : ≥ 52 mg/mL (225.82 mM) *"≥" means soluble, but saturation unknown. | ||
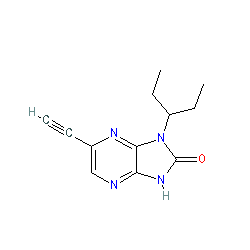
| Chemical Name | 5-ethynyl-3-pentan-3-yl-1H-imidazo[4,5-b]pyrazin-2-one | ||
| SMILES | CCC(CC)N1C2=NC(=CN=C2NC1=O)C#C | ||
| Standard InChIKey | RSQGZEAXODVTOL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H14N4O/c1-4-8-7-13-10-11(14-8)16(12(17)15-10)9(5-2)6-3/h1,7,9H,5-6H2,2-3H3,(H,13,15,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tirasemtiv is an activator of the fast skeletal muscle troponin complex.In Vitro:Tirasemtiv is a fast skeletal troponin activator that sensitizes the sarcomere to calcium; this mechanism of action amplifies the response of muscle to neuromuscular input producing greater force when nerve input is reduced. Tirasemtiv selectively sensitizes fast skeletal muscle troponin to calcium (Ca2+), and slows the rate of Ca2+ release from the regulatory troponin complex of fast skeletal muscle[1].In Vivo:A single dose of Tirasemtiv significantly increases submaximal isometric force, forelimb grip strength, grid hang time, and rotarod performance in a female transgenic mouse model (B6SJL-SOD1G93A) of ALS with functional deficits. Additionally, diaphragm force and tidal volume are significantly higher in Tirasemtiv-treated female B6SJL-SOD1G93A mice. At the 25% deficit milestone, vehicle-treated B6SJL-SOD1G93A mice demonstrated forelimb grip strength of 49.6±4.6 g. Tirasemtiv increases grip strength by 38% to 68.6±8.1g (p<0.05, single tailed t-test). Tirasemtiv doses of 2, 2, 2, and 4 mg/kg given at approximately 20 min intervals to achieve a cumulative dose of 10 mg/kg. Regression analysis of the log dose vs. response relationship indicated that Tirasemtiv significantly increased muscle force in WT and mid-stage B6SJL-SOD1G93A mice (WT p<0.0001; mid-stage p=0.0028). At later stages of disease, the mice exhibited a trend for increased muscle function in response to Tirasemtivcompared to baseline (p=0.064)[1]. References: | |||||

Tirasemtiv Dilution Calculator

Tirasemtiv Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3427 mL | 21.7136 mL | 43.4273 mL | 86.8546 mL | 108.5682 mL |
| 5 mM | 0.8685 mL | 4.3427 mL | 8.6855 mL | 17.3709 mL | 21.7136 mL |
| 10 mM | 0.4343 mL | 2.1714 mL | 4.3427 mL | 8.6855 mL | 10.8568 mL |
| 50 mM | 0.0869 mL | 0.4343 mL | 0.8685 mL | 1.7371 mL | 2.1714 mL |
| 100 mM | 0.0434 mL | 0.2171 mL | 0.4343 mL | 0.8685 mL | 1.0857 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tirasemtiv(CK 2017357) is a a small-molecule fast-skeletal-troponin activator, which is being developed as a potential treatment for diseases and conditions associated with aging, muscle weakness and wasting or neuromuscular dysfunction.
- LCL161
Catalog No.:BCC1691
CAS No.:1005342-46-0
- CVT 10216
Catalog No.:BCC5606
CAS No.:1005334-57-5
- Gelomulide N
Catalog No.:BCN6641
CAS No.:1005212-02-1
- Aeruginolactone
Catalog No.:BCN3695
CAS No.:1005208-88-7
- TCS 2002
Catalog No.:BCC6074
CAS No.:1005201-24-0
- Blasticidin A
Catalog No.:BCN1830
CAS No.:100513-53-9
- Sterigmatocystin
Catalog No.:BCN6885
CAS No.:10048-13-2
- Gastrin I (human)
Catalog No.:BCC5958
CAS No.:10047-33-3
- Rosiridin
Catalog No.:BCN5970
CAS No.:100462-37-1
- FFN 511
Catalog No.:BCC7799
CAS No.:1004548-96-2
- 1-EBIO
Catalog No.:BCC6904
CAS No.:10045-45-1
- Dihydroresveratrol 3-O-glucoside
Catalog No.:BCN5821
CAS No.:100432-87-9
- Monomethylsulochrin
Catalog No.:BCN7255
CAS No.:10056-14-1
- TC ASK 10
Catalog No.:BCC6301
CAS No.:1005775-56-3
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- NF 546
Catalog No.:BCC7804
CAS No.:1006028-37-0
- MK-2894
Catalog No.:BCC1757
CAS No.:1006036-87-8
- MK-2894 sodium salt
Catalog No.:BCC1758
CAS No.:1006036-88-9
- (-)-Epipinoresinol
Catalog No.:BCN3377
CAS No.:10061-38-8
- Desloratadine
Catalog No.:BCC4540
CAS No.:100643-71-8
- Ganoderenic acid A
Catalog No.:BCN3208
CAS No.:100665-40-5
- Ganoderenic acid B
Catalog No.:BCN7966
CAS No.:100665-41-6
- Ganoderenic acid C
Catalog No.:BCN3210
CAS No.:100665-42-7
- Ganoderenic acid D
Catalog No.:BCN2445
CAS No.:100665-43-8
Effect of tirasemtiv, a selective activator of the fast skeletal muscle troponin complex, in patients with peripheral artery disease.[Pubmed:24872402]
Vasc Med. 2014 Aug;19(4):297-306.
Tirasemtiv (CK-2017357), a novel small-molecule activator of the fast skeletal muscle troponin complex, slows the rate of calcium release from troponin, thus sensitizing fast skeletal muscle fibers to calcium. In preclinical studies, Tirasemtiv increased muscle force and delayed the onset and reduced the extent of muscle fatigue during hypoxia in vitro and muscle ischemia in situ. This study evaluated the effect of single doses of Tirasemtiv on measures of skeletal muscle function and fatigability in patients with stable calf claudication due to peripheral artery disease (PAD). Sixty-one patients with an ankle-brachial index Tirasemtiv 375 mg and 750 mg and matching placebo in random order about 1 week apart. After 33 patients were treated, the 750 mg dose was decreased to 500 mg due to adverse events and these dose groups were combined for analysis. On each study day, bilateral heel-raise testing was performed before and at 3 and 6 hours after dosing; a 6-minute walk test was performed at 4 hours after dosing. Claudicating calf muscle performance was increased at the highest dose and plasma concentration of Tirasemtiv; however, the 6-minute walk distance decreased with both the dose and plasma concentration of Tirasemtiv, possibly due to dose-related adverse events, particularly dizziness, that could impede walking ability. In conclusion, the mechanism of fast skeletal muscle troponin activation improved muscle function but not 6-minute walking distance in patients with claudication due to PAD. CLINICALTRIALSGOV IDENTIFIER NCT01131013:
A randomized, placebo-controlled, double-blind phase IIb trial evaluating the safety and efficacy of tirasemtiv in patients with amyotrophic lateral sclerosis.[Pubmed:26982815]
Amyotroph Lateral Scler Frontotemporal Degener. 2016 Jul-Aug;17(5-6):426-35.
Our objectives were to evaluate the safety and tolerability of Tirasemtiv over 12 weeks and its effect on the revised ALS Functional Rating Scale (ALSFRS-R) and other secondary functional measures. This randomized, double-blind, placebo-controlled trial enrolled adults with ALS and slow vital capacity (SVC) > 50% from 73 centers in eight countries. Patients who tolerated open-label Tirasemtiv 125 mg b.i.d. for one week were randomized to double-blind treatment either to placebo or Tirasemtiv, escalating to a maximum tolerated dose up to 250 mg b.i.d. The primary endpoint was the change from baseline in ALSFRS-R; secondary endpoints included SVC, maximum voluntary ventilation, sniff nasal inspiratory pressure, isometric muscle strength, and sub-maximum handgrip fatigue. Of 711 patients enrolled, 596 were randomized and received at least one dose of double-blind treatment. The primary endpoint showed no treatment effect (Tirasemtiv: -2.98 +/- 0.28, placebo: -2.40 +/- 0.25, p = 0.114); however, SVC and muscle strength declined significantly more slowly on Tirasemtiv (95% CI p = 0.0006, p = 0.0158, respectively). Dropouts and serious adverse events occurred more frequently in the Tirasemtiv group. In conclusion, this was a negative study with respect to the primary endpoint; however, the effects on SVC and muscle strength suggest a potentially important effect of Tirasemtiv warranting further evaluation over a longer period in ALS.
Fast skeletal muscle troponin activator tirasemtiv increases muscle function and performance in the B6SJL-SOD1G93A ALS mouse model.[Pubmed:24805850]
PLoS One. 2014 May 7;9(5):e96921.
Amyotrophic Lateral Sclerosis (ALS) is a motor neuron disease characterized by progressive motor neuron loss resulting in muscle atrophy, declining muscle function, and eventual paralysis. Patients typically die from respiratory failure 3 to 5 years from the onset of symptoms. Tirasemtiv is a fast skeletal troponin activator that sensitizes the sarcomere to calcium; this mechanism of action amplifies the response of muscle to neuromuscular input producing greater force when nerve input is reduced. Here, we demonstrate that a single dose of Tirasemtiv significantly increases submaximal isometric force, forelimb grip strength, grid hang time, and rotarod performance in a female transgenic mouse model (B6SJL-SOD1 G93A) of ALS with functional deficits. Additionally, diaphragm force and tidal volume are significantly higher in Tirasemtiv-treated female B6SJL-SOD1 G93A mice. These results support the potential of fast skeletal troponin activators to improve muscle function in neuromuscular diseases.
A Double-Blinded, Randomized, Placebo-Controlled Trial to Evaluate Efficacy, Safety, and Tolerability of Single Doses of Tirasemtiv in Patients with Acetylcholine Receptor-Binding Antibody-Positive Myasthenia Gravis.[Pubmed:25742919]
Neurotherapeutics. 2015 Apr;12(2):455-60.
Tirasemtiv is a fast skeletal troponin activator that sensitizes the sarcomere to calcium and increases muscle force following subtetanic nerve input. In an animal model of myasthenia gravis (MG), single oral doses of Tirasemtiv improved muscle force and reduced fatigability. The purpose of this study was to determine the effect of single doses of Tirasemtiv on skeletal muscle function and fatigability in patients with generalized MG. Thirty-two patients with acetylcholine receptor-antibody positive MG and muscle weakness received single doses of Tirasemtiv (250 mg or 500 mg) or placebo in a double-blind, randomized treatment sequence with each treatment separated by at least 1 week. Outcome measures included the Quantitative MG Score (QMG), MG Composite, Manual Muscle Testing, and forced vital capacity. At 6 h after dosing, Tirasemtiv produced dose-related improvements from baseline in the QMG score (slope: -0.49 QMG point per 250 mg; p = 0.02) and in percent predicted forced vital capacity (slope: 2.2% per 250 mg; p = 0.04). QMG improved >3 points in twice as many patients after 500 mg Tirasemtiv than after placebo. Both doses of Tirasemtiv were well tolerated; there were no premature terminations or serious adverse events. The results of this study suggest that Tirasemtiv may improve muscle function in MG and will be used to support further development of Tirasemtiv in neuromuscular diseases.


