AmifampridineCAS# 54-96-6 |
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 54-96-6 | SDF | Download SDF |
| PubChem ID | 5918 | Appearance | Powder |
| Formula | C5H7N3 | M.Wt | 109.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 3,4-Diaminopyridine | ||
| Solubility | DMSO : ≥ 38 mg/mL (348.21 mM) *"≥" means soluble, but saturation unknown. | ||
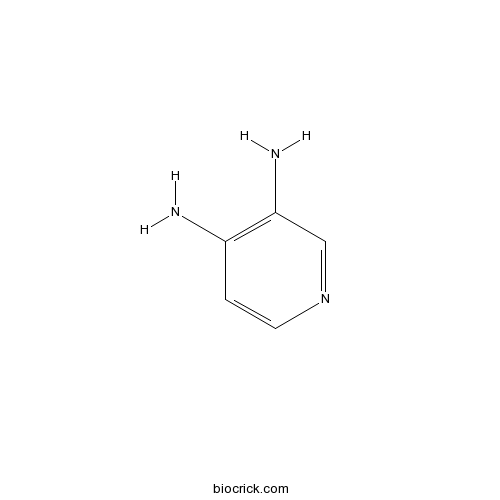
| Chemical Name | pyridine-3,4-diamine | ||
| SMILES | C1=CN=CC(=C1N)N | ||
| Standard InChIKey | OYTKINVCDFNREN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H7N3/c6-4-1-2-8-3-5(4)7/h1-3H,7H2,(H2,6,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Amifampridine is a drug, predominantly in the treatment of a number of rare muscle diseases.
Target: Others
Amifampridine is a drug, predominantly in the treatment of a number of rare muscle diseases. Amifampridine works by blocking potassium channel efflux in nerve terminals so that action potential duration is increased. Ca2+ channels can then be open for a longer time and allow greater acetylcholine release to stimulate muscle at the end plate. A 2005 systematic review from the Cochrane Collaboration found some data favouring its use in LEMS. Amifampridine is also used to treat many of the congenital myasthenic syndromes, particularly those with defects in choline acetyltransferase, downstream kinase 7, and those where any kind of defect causes fast channel behaviour of the acetylcholine receptor. From Wikipedia. References: | |||||

Amifampridine Dilution Calculator

Amifampridine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.1634 mL | 45.8169 mL | 91.6338 mL | 183.2677 mL | 229.0846 mL |
| 5 mM | 1.8327 mL | 9.1634 mL | 18.3268 mL | 36.6535 mL | 45.8169 mL |
| 10 mM | 0.9163 mL | 4.5817 mL | 9.1634 mL | 18.3268 mL | 22.9085 mL |
| 50 mM | 0.1833 mL | 0.9163 mL | 1.8327 mL | 3.6654 mL | 4.5817 mL |
| 100 mM | 0.0916 mL | 0.4582 mL | 0.9163 mL | 1.8327 mL | 2.2908 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Amifampridine is a drug, predominantly in the treatment of a number of rare muscle diseases.
- Pentylenetetrazole
Catalog No.:BCC7453
CAS No.:54-95-5
- Isoniazid
Catalog No.:BCC9003
CAS No.:54-85-3
- Cinanserin hydrochloride
Catalog No.:BCC6653
CAS No.:54-84-2
- Pilocarpine HCl
Catalog No.:BCC4702
CAS No.:54-71-7
- Idoxuridine
Catalog No.:BCC4666
CAS No.:54-42-2
- Metyrapone
Catalog No.:BCC7632
CAS No.:54-36-4
- Furosemide
Catalog No.:BCC3782
CAS No.:54-31-9
- Sodium salicylate
Catalog No.:BCC4846
CAS No.:54-21-7
- 5-Hydroxyindole-3-Acetic Acid
Catalog No.:BCC8285
CAS No.:54-16-0
- Tryptophan
Catalog No.:BCN2615
CAS No.:54-12-6
- L-Nicotine
Catalog No.:BCN6269
CAS No.:54-11-5
- Cefaclor
Catalog No.:BCC2527
CAS No.:53994-73-3
- Albendazole Oxide
Catalog No.:BCC4757
CAS No.:54029-12-8
- Etonogestrel
Catalog No.:BCC5230
CAS No.:54048-10-1
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
- Palosuran
Catalog No.:BCC4311
CAS No.:540769-28-6
- Isoastilbin
Catalog No.:BCN5719
CAS No.:54081-48-0
- 2-(1-Hydroxy-1-methylethyl)-4-methoxy-7H-furo[3,2-g][1]benzopyran-7-one
Catalog No.:BCN1422
CAS No.:54087-32-0
- L-Carnitine inner salt
Catalog No.:BCN1229
CAS No.:541-15-1
- Decamethonium Bromide
Catalog No.:BCC4568
CAS No.:541-22-0
- Isovaleramide
Catalog No.:BCC4668
CAS No.:541-46-8
- Muscone
Catalog No.:BCN6275
CAS No.:541-91-3
- 15-Hydroxydehydroabietic acid
Catalog No.:BCN5720
CAS No.:54113-95-0
- 9-Benzylcarbazole-3-carboxaldehyde
Catalog No.:BCC8800
CAS No.:54117-37-2
Genetic variation in aryl N-acetyltransferase results in significant differences in the pharmacokinetic and safety profiles of amifampridine (3,4-diaminopyridine) phosphate.[Pubmed:25692017]
Pharmacol Res Perspect. 2015 Feb;3(1):e00099.
The clinical use of Amifampridine phosphate for neuromuscular junction disorders is increasing. The metabolism of Amifampridine occurs via polymorphic aryl N-acetyltransferase (NAT), yet its pharmacokinetic (PK) and safety profiles, as influenced by this enzyme system, have not been investigated. The objective of this study was to assess the effect of NAT phenotype and genotype on the PK and safety profiles of Amifampridine in healthy volunteers (N = 26). A caffeine challenge test and NAT2 genotyping were used to delineate subjects into slow and fast acetylators for PK and tolerability assessment of single, escalating doses of Amifampridine (up to 30 mg) and in multiple daily doses (20 mg QID) of Amifampridine. The results showed that fast acetylator phenotypes displayed significantly lower C max, AUC, and shorter t 1/2 for Amifampridine than slow acetylators. Plasma concentrations of the N-acetyl metabolite were approximately twofold higher in fast acetylators. Gender differences were not observed. Single doses of Amifampridine demonstrated dose linear PKs. Amifampridine achieved steady state plasma levels within 1 day of dosing four times daily. No accumulation or time-dependent changes in Amifampridine PK parameters occurred. Overall, slow acetylators reported 73 drug-related treatment-emergent adverse events versus 6 in fast acetylators. Variations in polymorphic NAT corresponding with fast and slow acetylator phenotypes significantly affects the PK and safety profiles of Amifampridine.
Effects of Food Intake on the Relative Bioavailability of Amifampridine Phosphate Salt in Healthy Adults.[Pubmed:26101174]
Clin Ther. 2015 Jul 1;37(7):1555-63.
PURPOSE: Amifampridine (3,4-diaminopyridine) has been approved in the European Union for the treatment of Lambert-Eaton myasthenic syndrome. Amifampridine has a narrow therapeutic index, and supratherapeutic exposure has been associated with dose-dependent adverse events, including an increased risk for seizure. This study assessed the effect of food on the relative bioavailability of Amifampridine in healthy subjects and informed on conditions that can alter exposure. METHODS: This randomized, open-labeled, 2-treatment, 2-period crossover study enrolled 47 healthy male and female subjects. Subjects were randomly assigned to receive 2 single oral doses of Amifampridine phosphate salt (20 mg base equivalents per dose) under fed or fasted conditions separated by a washout period. Blood and urine samples for pharmacokinetic analyses were taken before and after dosing. Plasma concentrations of Amifampridine and an inactive 3-N-acetyl metabolite were determined. The relative bioavailability values of Amifampridine and metabolite were assessed based on the plasma PK parameters AUC0-infinity, AUC0-t, and Cmax in the fed and fasted states using noncompartmental pharmacokinetic analysis. Parent drug and metabolite excretion were calculated from urinary concentrations. A food effect on bioavailability would be established if the 90% CI of the ratio of population geometric mean value of AUC0-infinity, AUC0-t, or Cmax between fed and fasted administration was not within the bioequivalence range of 80% to 125%. Tolerability was assessed based on adverse-event reporting, clinical laboratory assessments, physical examination including vital sign measurements, 12-lead ECG, and concurrent medication use. FINDINGS: Food slowed and somewhat decreased the absorption of Amifampridine. There was a decrease in exposure (Cmax, 44%; AUC, 20%) after oral administration of Amifampridine phosphate salt in the presence of food, and mean Tmax was 2-fold longer in the fed state. The extent of exposure and plasma elimination half-life of the major metabolite was greater than those of Amifampridine in the fed and fasted conditions. Mean AUCs in the fed and fasted states were slightly greater in women than men, with no difference in mean Cmax. Orally administered Amifampridine was renally eliminated (>93%) as the parent compound and metabolite within 24 hours. Single oral doses of 20 mg of Amifampridine phosphate salt were considered well tolerated in both the fed and fasted conditions. High intersubject variability (%CVs, >30%) in Amifampridine pharmacokinetic parameter values was observed. IMPLICATIONS: At the intended dose under fasting conditions, Amifampridine exposure may be increased. European Union Drug Regulating Authorities Clinical Trials identifier: 2011-000596-13.
Amifampridine phosphate (Firdapse((R))) is effective and safe in a phase 3 clinical trial in LEMS.[Pubmed:26852139]
Muscle Nerve. 2016 May;53(5):717-25.
OBJECTIVE: We evaluated the efficacy and safety of Amifampridine phosphate (Firdapse((R))) for symptomatic treatment in Lambert-Eaton myasthenic syndrome (LEMS). METHODS: Phase 3, randomized, double-blind, study. Patients were treated initially with Amifampridine phosphate for 7-91 days, followed by randomization to continue Amifampridine phosphate for 14 days or placebo (7-day taper, 7-day placebo). The primary efficacy endpoints were changes from baseline at day 14 in Quantitative Myasthenia Gravis and Subject Global Impression scores. RESULTS: The coprimary efficacy end points and 1 of the secondary efficacy end points were met, showing a significant benefit of aminfampridine phosphate over placebo at Day 14. All 5 primary, secondary, and tertiary endpoints achieved statistical significance at Day 8. Amifampridine phosphate was well tolerated; the most common adverse events were oral and digital paresthesias, nausea, and headache. CONCLUSIONS: This study provides Class I evidence of efficacy of Amifampridine phosphate as a symptomatic treatment for LEMS.


