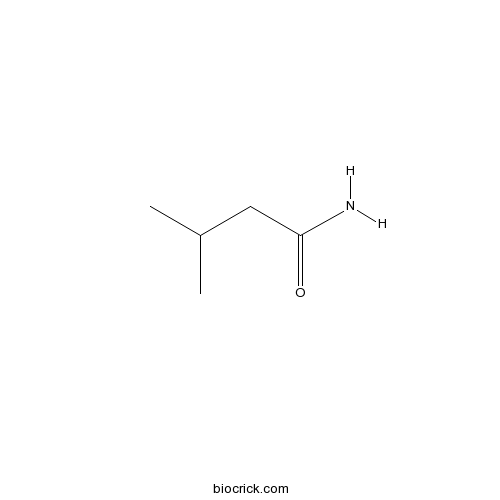
IsovaleramideCAS# 541-46-8 |
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- Mildronate
Catalog No.:BCC2289
CAS No.:76144-81-5
- Disulfiram
Catalog No.:BCC2098
CAS No.:97-77-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 541-46-8 | SDF | Download SDF |
| PubChem ID | 10930 | Appearance | Powder |
| Formula | C5H11NO | M.Wt | 101.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 3-Methylbutanamide | ||
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | 3-methylbutanamide | ||
| SMILES | CC(C)CC(=O)N | ||
| Standard InChIKey | SANOUVWGPVYVAV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H11NO/c1-4(2)3-5(6)7/h4H,3H2,1-2H3,(H2,6,7) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isovaleramide is an active principle on central nervous system from Valeriana pavonii, as an anticonvulsant. Target: in vitro: Isovaleramide (300 μM) exhibits a 42% of inhibition of the binding of 3H-FNZ to its sites. in vivo: Isovaleramide at 100 mg/Kg, p.o, evidences a 90% index protection against the maximal electroshock seizure in mice (MES). | |||||

Isovaleramide Dilution Calculator

Isovaleramide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.8863 mL | 49.4315 mL | 98.8631 mL | 197.7261 mL | 247.1577 mL |
| 5 mM | 1.9773 mL | 9.8863 mL | 19.7726 mL | 39.5452 mL | 49.4315 mL |
| 10 mM | 0.9886 mL | 4.9432 mL | 9.8863 mL | 19.7726 mL | 24.7158 mL |
| 50 mM | 0.1977 mL | 0.9886 mL | 1.9773 mL | 3.9545 mL | 4.9432 mL |
| 100 mM | 0.0989 mL | 0.4943 mL | 0.9886 mL | 1.9773 mL | 2.4716 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Isovaleramide is an anticonvulsant molecule isolated from Valeriana pavonii, it inhibits the liver alcohol dehydrogenases.
- Decamethonium Bromide
Catalog No.:BCC4568
CAS No.:541-22-0
- L-Carnitine inner salt
Catalog No.:BCN1229
CAS No.:541-15-1
- 2-(1-Hydroxy-1-methylethyl)-4-methoxy-7H-furo[3,2-g][1]benzopyran-7-one
Catalog No.:BCN1422
CAS No.:54087-32-0
- Isoastilbin
Catalog No.:BCN5719
CAS No.:54081-48-0
- Palosuran
Catalog No.:BCC4311
CAS No.:540769-28-6
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
- Etonogestrel
Catalog No.:BCC5230
CAS No.:54048-10-1
- Albendazole Oxide
Catalog No.:BCC4757
CAS No.:54029-12-8
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Pentylenetetrazole
Catalog No.:BCC7453
CAS No.:54-95-5
- Isoniazid
Catalog No.:BCC9003
CAS No.:54-85-3
- Cinanserin hydrochloride
Catalog No.:BCC6653
CAS No.:54-84-2
- Muscone
Catalog No.:BCN6275
CAS No.:541-91-3
- 15-Hydroxydehydroabietic acid
Catalog No.:BCN5720
CAS No.:54113-95-0
- 9-Benzylcarbazole-3-carboxaldehyde
Catalog No.:BCC8800
CAS No.:54117-37-2
- Apoptosis Inhibitor
Catalog No.:BCC1143
CAS No.:54135-60-3
- Neoisoastilbin
Catalog No.:BCN6532
CAS No.:54141-72-9
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Vicriviroc Malate
Catalog No.:BCC1230
CAS No.:541503-81-5
- Apilimod
Catalog No.:BCC5286
CAS No.:541550-19-0
- 1-Monopalmitin
Catalog No.:BCN7749
CAS No.:542-44-9
- Cimaterol
Catalog No.:BCC6647
CAS No.:54239-37-1
- 3-Benzoylpyridine
Catalog No.:BCC8623
CAS No.:5424-19-1
- Stachyose trihydrate
Catalog No.:BCN8361
CAS No.:54261-98-2
Synthesis of N4-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)-L-asparagine analogues. n-Butyramide, 3-chloropropionamide, 3-aminopropionamide, and isovaleramide analogues.[Pubmed:11398986]
Carbohydr Res. 2001 Apr 23;331(4):439-44.
The syntheses of four analogues of N4-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)-L-asparagine are described. Activated carboxylic acids were reacted with 2-acetamido-2-deoxy-beta-D-glucopyranosylamine. n-Butyric anhydride gave N-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)-n-butyramide. 3-Chloropropionic anhydride was synthesized from 3-chloropropionic acid and gave N-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)-3-chloropropionamide. Equilibration of the latter with ammonium bicarbonate gave N1-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)-3-aminopropionamide. Succinimidyl isovalerate was synthesized and gave N-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)-Isovaleramide.
[Isovaleramide, an anticonvulsant molecule isolated from Valeriana pavonii].[Pubmed:20890571]
Biomedica. 2010 Apr-Jun;30(2):245-50.
INTRODUCTION: Fractioning of an extract of Valeriana pavonii, a native species used in Colombian folk medicine as tranquilizer, led to the isolation and identification of Isovaleramide, one of the active constituents responsible for its central nervous system activity as anticonvulsant. OBJECTIVE: Description of the isolation and identification of Isovaleramide, an active principle on central nervous system from Valeriana pavonii. MATERIALS AND METHODS: The purification of Isovaleramide was carried out by chromatographic techniques. Its structural elucidation was determined by nuclear magnetic resonance and mass spectrometry. Maximal electroshock seizure was used as in vivo pharmacological test, additionally in vitro GABA-A/BDZ-binding site studies were performed. RESULTS: Isovaleramide was isolated from the most active fraction of Valeriana pavonii. This compound, at 100 mg/Kg, p.o, evidenced a 90% index protection against the maximal electroshock seizure in mice (MES), comparable to the reference agent: sodium phenytoin (20 mg/kg, p.o, 100%). In the in vitro assay, Isovaleramide (300 microM) exhibited a 42% of inhibition of the binding of (3)H-FNZ to its sites. CONCLUSION: Isovaleramide is one of the active anticonvulsant constituents of Valeriana pavonii, for the first time reported in this species. These results support the traditional use of Valeriana pavonii and its interest as a therapeutic source.


