3-BenzoylpyridineCAS# 5424-19-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5424-19-1 | SDF | Download SDF |
| PubChem ID | 21540 | Appearance | Powder |
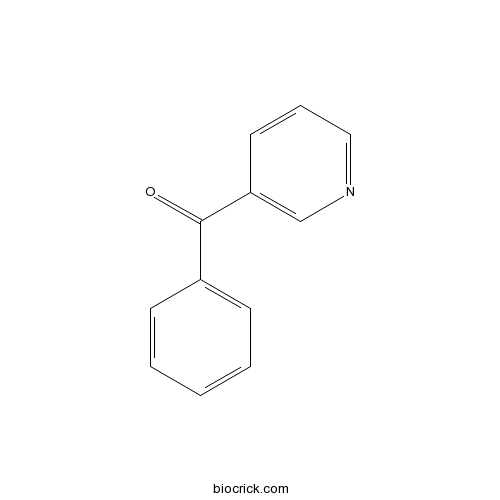
| Formula | C12H9NO | M.Wt | 183 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | phenyl(pyridin-3-yl)methanone | ||
| SMILES | C1=CC=C(C=C1)C(=O)C2=CN=CC=C2 | ||
| Standard InChIKey | RYMBAPVTUHZCNF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H9NO/c14-12(10-5-2-1-3-6-10)11-7-4-8-13-9-11/h1-9H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-Benzoylpyridine Dilution Calculator

3-Benzoylpyridine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4645 mL | 27.3224 mL | 54.6448 mL | 109.2896 mL | 136.612 mL |
| 5 mM | 1.0929 mL | 5.4645 mL | 10.929 mL | 21.8579 mL | 27.3224 mL |
| 10 mM | 0.5464 mL | 2.7322 mL | 5.4645 mL | 10.929 mL | 13.6612 mL |
| 50 mM | 0.1093 mL | 0.5464 mL | 1.0929 mL | 2.1858 mL | 2.7322 mL |
| 100 mM | 0.0546 mL | 0.2732 mL | 0.5464 mL | 1.0929 mL | 1.3661 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cimaterol
Catalog No.:BCC6647
CAS No.:54239-37-1
- 1-Monopalmitin
Catalog No.:BCN7749
CAS No.:542-44-9
- Apilimod
Catalog No.:BCC5286
CAS No.:541550-19-0
- Vicriviroc Malate
Catalog No.:BCC1230
CAS No.:541503-81-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Neoisoastilbin
Catalog No.:BCN6532
CAS No.:54141-72-9
- Apoptosis Inhibitor
Catalog No.:BCC1143
CAS No.:54135-60-3
- 9-Benzylcarbazole-3-carboxaldehyde
Catalog No.:BCC8800
CAS No.:54117-37-2
- 15-Hydroxydehydroabietic acid
Catalog No.:BCN5720
CAS No.:54113-95-0
- Muscone
Catalog No.:BCN6275
CAS No.:541-91-3
- Isovaleramide
Catalog No.:BCC4668
CAS No.:541-46-8
- Decamethonium Bromide
Catalog No.:BCC4568
CAS No.:541-22-0
- Stachyose trihydrate
Catalog No.:BCN8361
CAS No.:54261-98-2
- (+)-Fluprostenol
Catalog No.:BCC7947
CAS No.:54276-17-4
- 2',4'-Dihydroxy-3',6'-dimethoxydihydrochalcone
Catalog No.:BCN1421
CAS No.:54299-52-4
- Ac-Gly-OH
Catalog No.:BCC2943
CAS No.:543-24-8
- Amsacrine hydrochloride
Catalog No.:BCC4310
CAS No.:54301-15-4
- Protopseudohypericin
Catalog No.:BCN2813
CAS No.:54328-09-5
- 4beta-Hydroxywithanolide E
Catalog No.:BCN7572
CAS No.:54334-04-2
- Eriodictyol 7,3'-dimethyl ether
Catalog No.:BCN8105
CAS No.:54352-60-2
- Decarine
Catalog No.:BCN5721
CAS No.:54354-62-0
- 4'-Methoxyacetoacetanilide
Catalog No.:BCC8712
CAS No.:5437-98-9
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- 7-Hydroxy-5,8-dimethoxyflavanone
Catalog No.:BCN5722
CAS No.:54377-24-1
Comparative induction of CYP3A and CYP2B in rat liver by 3-benzoylpyridine and metyrapone.[Pubmed:9717516]
Chem Biol Interact. 1998 Jun 5;113(3):161-73.
3-Benzoylpyridine (3BP) is a major metabolite of HGG-12, and oxime that has been synthesized as a potential antidote to the toxic effects of soman and other anticholinesterases. Structural similarities exist between 3BP, the cytochrome P450 (CYP)-inducer metyrapone (MET) and other 3-substituted pyridines that interact with CYPs. The present study evaluated the regulatory effects of 3BP on CYP expression in rat liver. Both 3BP and MET (100 mg/kg) increased total hepatic microsomal holo-CYP content significantly 24 h after administration to male rats. Pronounced increases in activities mediated by CYP2B (androstenedione 16 beta-hydroxylation and 7-pentylresorufin O-depentylation) were produced by 3BP and MET, which correlated with respective 9- and 14-fold increases in CYP2B immunoreactive protein. In addition, both agents slightly increased rates of microsomal CYP3A-dependent steroid 6 beta-hydroxylation, troleandomycin metabolite complex formation and total CYP3A immunoreactive protein. Induction of the dexamethasone-inducible CYP3A23 mRNA to 4.5- and 2.5-fold of control was detected in liver of MET- and 3BP-induced rats; CYP3A2 mRNA levels were unchanged. Analogous in vitro studies revealed that MET was a preferential inhibitor of CYP3A-mediated steroid 6 beta-hydroxylation activity, but 3BP was inactive against constitutive steroid hydroxylase CYPs. These findings indicate that the structurally related 3BP and MET elicit similar induction effects on CYPs 2B and 3A23 in rat liver after in vivo administration, but differential inhibitory effects of the chemicals on CYP activity in vitro. Recent reports have implicated a microsomal binding site in the induction of CYP3A1/3A23 in rat liver. In light of the present findings, substituted pyridines like 3BP may be useful tools in structure-activity studies to evaluate the physicochemical requirements for binding to this protein.
Dependence of the substrate specificity and kinetic mechanism of horse-liver alcohol dehydrogenase on the size of the C-3 pyridinium substituent. 3-Benzoylpyridine-adenine dinucleotide.[Pubmed:3758068]
Eur J Biochem. 1986 Sep 1;159(2):375-80.
The kinetic mechanism and the substrate specificity of liver alcohol dehydrogenase are changed when 3-Benzoylpyridine-adenine dinucleotide is used as coenzyme. Only primary alcohols are substrates of the enzyme and with ethanol the mechanism becomes rapid-equilibrium random bi-bi. According to model building experiments on a graphic display, the benzoyl group partially enters the substrate binding site, whereas the essential interactions between coenzyme and enzyme are preserved. This restraint on the substrate binding site provides a molecular explanation for the observed dependence between coenzyme and substrate chemical structures.
The metabolism of 3-benzoylpyridine.[Pubmed:6673376]
Xenobiotica. 1983 Nov;13(11):649-59.
3-Benzoylpyridine (3-BP), a decomposition product of the soman antidote, HGG-12 (3-benzoylpyridino(1)-methyl 2'-hydroxyiminomethylpyridino(1')methyl ether dichloride) was rapidly metabolized in the isolated perfused rat liver, giving 3-(alpha-hydroxybenzyl)pyridine and its corresponding glucuronide, 3-Benzoylpyridine-N-oxide, and 3-(alpha-hydroxybenzyl)pyridine-N-oxide. The latter is formed both from 3-(alpha-hydroxybenzyl)pyridine and 3-Benzoylpyridine-N-oxide. Metabolism of 3-BP studied in rats and dogs in vivo revealed significant species differences. In rat, 80% of 14C-3-BP was excreted as N-oxides and alpha-hydroxybenzyl derivatives in the urine. In dogs, 95% dose was excreted in urine mostly as the glucuronide of 3-(alpha-hydroxybenzyl)pyridine and as the quaternary pyridinium compounds, 3-benzoyl-1-methylpyridinium and 3-(alpha-hydroxybenzyl)-1-methylpyridinium. These latter were hardly detected in rat urine. In contrast to rats, the N-oxides were present only in small amounts in dog urine.


