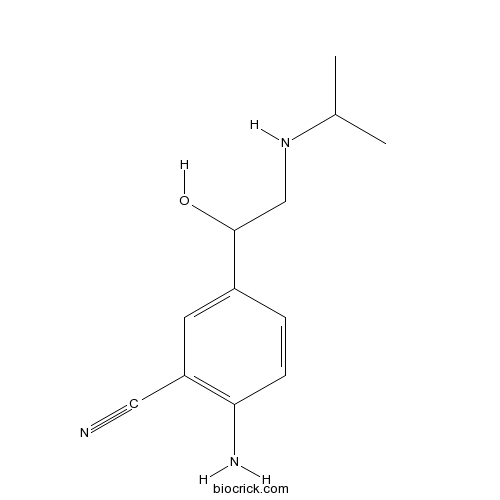
CimaterolCAS# 54239-37-1 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
- Nesbuvir
Catalog No.:BCC1796
CAS No.:691852-58-1
- PSI-6206
Catalog No.:BCC3609
CAS No.:863329-66-2
- RO-9187
Catalog No.:BCC1904
CAS No.:876708-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 54239-37-1 | SDF | Download SDF |
| PubChem ID | 2755 | Appearance | Powder |
| Formula | C12H17N3O | M.Wt | 219.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol and to 100 mM in DMSO | ||
| Chemical Name | 2-amino-5-[1-hydroxy-2-(propan-2-ylamino)ethyl]benzonitrile | ||
| SMILES | CC(C)NCC(C1=CC(=C(C=C1)N)C#N)O | ||
| Standard InChIKey | BUXRLJCGHZZYNE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H17N3O/c1-8(2)15-7-12(16)9-3-4-11(14)10(5-9)6-13/h3-5,8,12,15-16H,7,14H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | β-Adrenergic agonist. |

Cimaterol Dilution Calculator

Cimaterol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5602 mL | 22.8009 mL | 45.6017 mL | 91.2034 mL | 114.0043 mL |
| 5 mM | 0.912 mL | 4.5602 mL | 9.1203 mL | 18.2407 mL | 22.8009 mL |
| 10 mM | 0.456 mL | 2.2801 mL | 4.5602 mL | 9.1203 mL | 11.4004 mL |
| 50 mM | 0.0912 mL | 0.456 mL | 0.912 mL | 1.8241 mL | 2.2801 mL |
| 100 mM | 0.0456 mL | 0.228 mL | 0.456 mL | 0.912 mL | 1.14 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-Monopalmitin
Catalog No.:BCN7749
CAS No.:542-44-9
- Apilimod
Catalog No.:BCC5286
CAS No.:541550-19-0
- Vicriviroc Malate
Catalog No.:BCC1230
CAS No.:541503-81-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Neoisoastilbin
Catalog No.:BCN6532
CAS No.:54141-72-9
- Apoptosis Inhibitor
Catalog No.:BCC1143
CAS No.:54135-60-3
- 9-Benzylcarbazole-3-carboxaldehyde
Catalog No.:BCC8800
CAS No.:54117-37-2
- 15-Hydroxydehydroabietic acid
Catalog No.:BCN5720
CAS No.:54113-95-0
- Muscone
Catalog No.:BCN6275
CAS No.:541-91-3
- Isovaleramide
Catalog No.:BCC4668
CAS No.:541-46-8
- Decamethonium Bromide
Catalog No.:BCC4568
CAS No.:541-22-0
- L-Carnitine inner salt
Catalog No.:BCN1229
CAS No.:541-15-1
- 3-Benzoylpyridine
Catalog No.:BCC8623
CAS No.:5424-19-1
- Stachyose trihydrate
Catalog No.:BCN8361
CAS No.:54261-98-2
- (+)-Fluprostenol
Catalog No.:BCC7947
CAS No.:54276-17-4
- 2',4'-Dihydroxy-3',6'-dimethoxydihydrochalcone
Catalog No.:BCN1421
CAS No.:54299-52-4
- Ac-Gly-OH
Catalog No.:BCC2943
CAS No.:543-24-8
- Amsacrine hydrochloride
Catalog No.:BCC4310
CAS No.:54301-15-4
- Protopseudohypericin
Catalog No.:BCN2813
CAS No.:54328-09-5
- 4beta-Hydroxywithanolide E
Catalog No.:BCN7572
CAS No.:54334-04-2
- Eriodictyol 7,3'-dimethyl ether
Catalog No.:BCN8105
CAS No.:54352-60-2
- Decarine
Catalog No.:BCN5721
CAS No.:54354-62-0
- 4'-Methoxyacetoacetanilide
Catalog No.:BCC8712
CAS No.:5437-98-9
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
Efficient synthesis of D6 -clenproperol and D6 -cimaterol using deuterium isopropylamine as labelled precursor.[Pubmed:27753133]
J Labelled Comp Radiopharm. 2016 Nov;59(13):552-556.
This report presents an efficient synthesis of D6 -clenproperol and D6 -Cimaterol with 99.5% and 99.7% isotopic abundance in acceptable yields and excellent chemical purities with deuterium isopropylamine as labelled precursor. Their structures and the isotope-abundance were confirmed by proton nuclear magnetic resonance and liquid chromatography-mass spectrometry.
Determination of clenbuterol, salbutamol, and cimaterol in bovine retina by electrospray ionization-liquid chromatography-tandem mass spectrometry.[Pubmed:15084084]
J AOAC Int. 2004 Jan-Feb;87(1):31-8.
An electrospray ionization-liquid chromatography-tandem mass spectrometry (ESI/LC/MS/MS) method was developed for the simultaneous determination of the beta-agonists clenbuterol, salbutamol, and Cimaterol in bovine retina. The tissue was homogenized in alkaline buffer and spiked to give 10, 15, and 20 ng/g each of the 3 analytes together with the internal standards d6-salbutamol and d6-clenbuterol. The mixture was incubated with protease enzyme to release any protein-bound analytes and then made alkaline before extraction with isobutanol. The extract was dissolved in water and transferred to a clenbuterol immunoaffinity column. After washing, the analytes were eluted and analyzed by ESI/LC/MS/MS using a C18 column with acetic acid-methanol as mobile phase. No interferences were observed from the spiked retina extract at the various single-reaction monitoring modes. Average recoveries for clenbuterol, salbutamol, and Cimaterol were 94, 85, and 87% with coefficients of variation (CVs) of 9.4, 9.9, and 8.6%, respectively. A correlation coefficient of r2 = 0.9999 was obtained for all analytes. The limits of detection for clenbuterol, salbutamol, and Cimaterol, determined from 3 times the standard deviation of 7 replicates of the lowest spike, were 2.5, 3.5, and 2.0 ng/g with CVs of 8.9, 11.6, and 7.2%, respectively.
[Simultaneous determination of cimaterol, clenbuterol and salbutamol in feeds by capillary zone electrophoresis].[Pubmed:16124569]
Se Pu. 2005 May;23(3):261-3.
A method for simultaneous determination of Cimaterol, clenbuterol and salbutamol in feeds was developed by capillary zone electrophoresis. The effects of the experimental conditions on the separation and determination of Cimaterol, clenbuterol and salbutamol have been examined. Under the optimum conditions, all three compounds were completely separated in 8 min. The linear range of Cimaterol, clenbuterol and salbutamol was 0.1-1.0 mg/L, and the detection limits (S/N = 3) were 0.02, 0.03 and 0.02 mg/L, respectively. The developed method has been used for the determination of spiked feed samples. The recoveries of Cimaterol, clenbuterol and salbutamol were 89%-103%, 86%-91% and 83%-87%, respectively. The relative standard deviations for Cimaterol, clenbuterol and salbutamol were 3.8%-4.3%, 3.6%-5.0% and 4.0%-5.6%, respectively. The proposed method is a sensitive, fast and simple method for the determination of Cimaterol, clenbuterol and salbutamol in feeds.
Field-amplified on-line sample stacking for separation and determination of cimaterol, clenbuterol and salbutamol using capillary electrophoresis.[Pubmed:16828108]
J Chromatogr A. 2006 Aug 25;1125(1):124-8.
A capillary electrophoresis method, using field-amplified sample injection (FASI), was developed for separation and determination of some beta 2-agonists, such as Cimaterol, clenbuterol and salbutamol. The optimum conditions for this system had been investigated in detail. The precision of the migration time, peak height and accuracy were determined in both intra-day (n = 5) and inter-day (n = 15) assays. Under the optimum conditions, the detection limits (defined as S/N = 3) of this method were found to be lower than 2.0 ng/mL for all of these three beta 2-agonists, which were much lower than that of the conventional electro-migration injection method, the enhancement factors were greatly improved to be 30-40-fold. Such lower detection limit lets this method to be suitable for determination of above-mentioned beta 2-agonists in the urine sample. The mean recoveries in urine were higher than 96.2%, 95.6% and 95.3% for Cimaterol, clenbuterol and salbutamol, respectively, with relative standard deviations lower than 3.5%.
Function and regulation of the beta 3-adrenoceptor.[Pubmed:8979772]
Trends Pharmacol Sci. 1996 Oct;17(10):373-81.
The cloning, sequencing and expression in model systems of the previously unidentified beta 3-adrenoceptor recently led to an extensive functional characterization. Ligand binding and adenylate cyclase activation studies helped define a specific profile that is quite distinct from that of the beta 1- and beta 2-adrenoceptors, but strongly reminiscent of most of the 'atypical' beta-adrenoceptor-mediated responses reported in earlier pharmacological studies. More recently, a naturally occurring variation in the human beta 3-adrenoceptor has been correlated with hereditary obesity and with increased dynamic capacity to add on weight and develop non-insulin dependent diabetes in Western obese patients. Donny Strosberg and France Pietri-Rouxel describe how results now provide a consistent picture of an important role for the human beta 3-adrenoceptor in the regulation of lipid metabolism and as an obvious target for drugs to treat some forms of obesity and diabetes.
Structure-activity relationships in further series of amino-halogen substituted phenyl-aminoethanols.[Pubmed:6152155]
Arzneimittelforschung. 1984;34(11A):1625-32.
Series of 1-(4-amino-phenyl)-2-aminoethanol derivatives having different substituents in the phenyl nucleus were compared with 1-(4-amino-3,5-dichloro-phenyl)-2-tert.-butylamino-ethanol (clenbuterol) with regard to their action on the adrenergic beta-receptors of guinea pigs, cats and rats. The exchange of the two chlorine atoms of clenbuterol led to substances with a potent beta 2-mimetic activity, surpassing that of clenbuterol, in the bronchial muscles and/or to a beta 1-blocking action in the heart with a relatively low beta 1-intrinsic activity. The strongest action was found with the chloro-cyano, fluoro-cyano and mono-cyano substituted compounds. The chloro-fluoro, bromo-fluoro, mono-fluoro and chloro-trifluoromethyl substituted compounds showed a good oral absorption, comparable to that of clenbuterol. Because of a particularly low beta 1-intrinsic activity in the heart with a long-lasting beta 2-mimetic action on the bronchial muscles also after oral administration, the 1-(4-amino-3-chloro-5-trifluoromethyl-phenyl)-2-tert.-butylamino-ethanol hydrochloride (mabuterol) must come of all the structures investigated particularly into consideration as broncholytic agent for therapeutic use.


