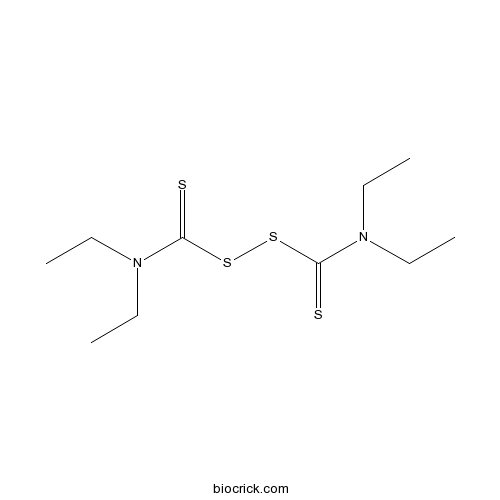
DisulfiramInhibits expression of MMP-2 and MMP-9; displays a range of other activities CAS# 97-77-8 |
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- Mildronate
Catalog No.:BCC2289
CAS No.:76144-81-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 97-77-8 | SDF | Download SDF |
| PubChem ID | 3117 | Appearance | Powder |
| Formula | C10H20N2S4 | M.Wt | 296.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Tetraethylthiuram disulfide | ||
| Solubility | DMSO : 75 mg/mL (252.92 mM; Need ultrasonic) | ||
| Chemical Name | diethylcarbamothioylsulfanyl N,N-diethylcarbamodithioate | ||
| SMILES | CCN(CC)C(=S)SSC(=S)N(CC)CC | ||
| Standard InChIKey | AUZONCFQVSMFAP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H20N2S4/c1-5-11(6-2)9(13)15-16-10(14)12(7-3)8-4/h5-8H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of aldehyde dehydrogenase that displays antialcoholism activity. Shown to reversibly stimulate Ca2+-ATPase activity and inhibit V-ATPase (EC50 = 26 μM). Also inhibits expression of MMP-2 and MMP-9 and displays anti-invasive activity. |

Disulfiram Dilution Calculator

Disulfiram Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3722 mL | 16.8611 mL | 33.7223 mL | 67.4445 mL | 84.3057 mL |
| 5 mM | 0.6744 mL | 3.3722 mL | 6.7445 mL | 13.4889 mL | 16.8611 mL |
| 10 mM | 0.3372 mL | 1.6861 mL | 3.3722 mL | 6.7445 mL | 8.4306 mL |
| 50 mM | 0.0674 mL | 0.3372 mL | 0.6744 mL | 1.3489 mL | 1.6861 mL |
| 100 mM | 0.0337 mL | 0.1686 mL | 0.3372 mL | 0.6744 mL | 0.8431 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Disulfiram blocks the processing of alcohol in the body by inhibiting acetaldehyde dehydrogenase thus causing an unpleasant reaction when alcohol is consumed.
- Ac-Ala-OH
Catalog No.:BCC3189
CAS No.:97-69-8
- 2-Methylvaleric acid
Catalog No.:BCN8498
CAS No.:97-61-0
- Allantoin
Catalog No.:BCN4527
CAS No.:97-59-6
- Solvent Yellow 3
Catalog No.:BCC9150
CAS No.:97-56-3
- Isoeugenol
Catalog No.:BCN8312
CAS No.:97-54-1
- Eugenol
Catalog No.:BCN5964
CAS No.:97-53-0
- DTG
Catalog No.:BCC6812
CAS No.:97-39-2
- Momordin Ic
Catalog No.:BCN1216
CAS No.:96990-18-0
- Cisatracurium Besylate
Catalog No.:BCC4345
CAS No.:96946-42-8
- Artanin
Catalog No.:BCN4517
CAS No.:96917-26-9
- Cyproheptadine hydrochloride
Catalog No.:BCC5161
CAS No.:969-33-5
- 1,2-Bis(4'-methyl-2,2'-bipyridin-4-yl)ethane
Catalog No.:BCC8414
CAS No.:96897-04-0
- (+)-Gallocatechin
Catalog No.:BCN5928
CAS No.:970-73-0
- (-)-Epigallocatechin(EGC)
Catalog No.:BCN4519
CAS No.:970-74-1
- Porfimer Sodium
Catalog No.:BCC5353
CAS No.:97067-70-4
- AMN 082 dihydrochloride
Catalog No.:BCC7344
CAS No.:97075-46-2
- 6-Geranylnaringenin
Catalog No.:BCN3001
CAS No.:97126-57-3
- 6-Epi-8-O-acetylharpagide
Catalog No.:BCN4550
CAS No.:97169-44-3
- 3-Ethoxyandrosta-3,5-dien-17-one
Catalog No.:BCC8630
CAS No.:972-46-3
- Meisoindigo
Catalog No.:BCC5132
CAS No.:97207-47-1
- Eriobofuran
Catalog No.:BCN7436
CAS No.:97218-06-9
- Picfeltarraenin IB
Catalog No.:BCN2845
CAS No.:97230-46-1
- Picfeltarraenin IA
Catalog No.:BCN1041
CAS No.:97230-47-2
- Topiramate
Catalog No.:BCC2314
CAS No.:97240-79-4
Disulfiram-loaded porous PLGA microparticle for inhibiting the proliferation and migration of non-small-cell lung cancer.[Pubmed:28182125]
Int J Nanomedicine. 2017 Jan 24;12:827-837.
In this study, poly(lactic-co-glycolic acid) (PLGA) was used as a carrier to construct Disulfiram-loaded porous microparticle through the emulsion solvent evaporation method, using ammonium bicarbonate as a porogen. The microparticle possessed highly porous surface, suitable aerodynamic diameter for inhalation (8.31+/-1.33 microm), favorable drug loading (4.09%+/-0.11%), and sustained release profile. The antiproliferation effect of release supernatant was detected through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay using non-small-cell lung cancer A549 as a model, with only 13.3% of cell viability observed for the release supernatant at 7 days. The antiproliferation mechanism was elucidated to be associated with the enhanced induction of cell apoptosis and cell cycle arrest at S phase through flow cytometry and Western blotting analysis. Finally, wound healing and transwell migration assay showed that they could efficiently inhibit the cell migration. These results demonstrated that Disulfiram-loaded porous PLGA microparticle could achieve favorable antitumor efficiency, implying the potential of treating non-small-cell lung cancer in a pulmonary administration.
Repositioning disulfiram as a radiosensitizer against atypical teratoid/rhabdoid tumor.[Pubmed:28340172]
Neuro Oncol. 2017 Aug 1;19(8):1079-1087.
Background: Atypical teratoid/rhabdoid tumor (AT/RT) is one of the most common malignant brain tumors in infants. Although cancer stem cells of AT/RT express aldehyde dehydrogenase (ALDH), effective chemotherapies against AT/RT have not been established. Here, we examined radiosensitizing effects of Disulfiram (DSF), an irreversible inhibitor of ALDH against AT/RT for a novel therapeutic method. Methods: Patient-derived primary cultured AT/RT cells (SNU.AT/RT-5 and SNU.AT/RT-6) and established AT/RT cell lines (BT-12 and BT-16) were used to assess therapeutic effects of combining DSF with radiation treatment (RT). Survival fraction by clonogenic assay, protein expression, immunofluorescence, and autophagy analysis were evaluated in vitro. Antitumor effects of combining DSF with RT were verified by bioluminescence imaging, tumor volume, and survival analysis in vivo. Results: The results demonstrated that DSF at low concentration enhanced the radiosensitivity of AT/RT cells with reduction of survival fraction to 1.211.58. DSF increased DNA double-strand break (gamma-H2AX, p-DNA-PKcs, and p-ATM), apoptosis (cleaved caspase-3), autophagy (LC3B), and cell cycle arrest (p21) in irradiated AT/RT cells, while it decreased anti-apoptosis (nuclear factor-kappaB, Survivin, and B-cell lymphoma 2 [Bcl2]). In vivo, DSF and RT combined treatment significantly reduced tumor volumes and prolonged the survival of AT/RT mouse models compared with single treatments. The combined treatment also increased gamma-H2AX, cleaved caspase-3, and LC3B expression and decreased ALDH1, Survivin, and Bcl2 expression in vivo. Conclusions: DSF and RT combination therapy has additive therapeutic effects on AT/RT by potentiating programmed cell death, including apoptosis and autophagy of AT/RT cells. We suggest that DSF can be applied as a radiosensitizer in AT/RT treatment.
Disulfiram overcomes bortezomib and cytarabine resistance in Down-syndrome-associated acute myeloid leukemia cells.[Pubmed:28143565]
J Exp Clin Cancer Res. 2017 Feb 1;36(1):22.
BACKGROUND: Children with Down syndrome (DS) have increased risk for developing AML (DS-AMKL), and they usually experience severe therapy-related toxicities compared to non DS-AMKL. Refractory/relapsed disease has very poor outcome, and patients would benefit from novel, less toxic, therapeutic strategies that overcome resistance. Relapse/resistance are linked to cancer stem cells with high aldehyde dehydrogenase (ALDH) activity. The purpose of the present work was to study less toxic alternative therapeutic agents for relapsed/refractory DS-AMKL. METHODS: Fourteen AML cell lines including the DS-AMKL CMY and CMK from relapsed/refractory AML were used. Cytarabine (Ara-C), bortezomib (BTZ), Disulfiram/copper (DSF/Cu(2+)) were evaluated for cytotoxicity, depletion of ALDH-positive cells, and resistance. BTZ-resistant CMY and CMK variants were generated by continuous BTZ treatment. Cell viability was assessed using CellTiter-Glo(R), ALDH activity by ALDELUOR(TM), and proteasome inhibition by western blot of ubiquitinated proteins and the Proteasome-Glo Chymotrypsin-Like (CT-like) assay, apoptosis by Annexin V Fluos/Propidium iodide staining, and mutations were detected using PCR, cloning and sequencing. RESULTS: Ara-C-resistant AML cell lines were sensitive to BTZ and DSF/Cu(2+). The Ara-C-resistant DS-AMKL CMY cells had a high percentage of ALDH(bright) "stem-like" populations that may underlie Ara-C resistance. One percent of these cells were still resistant to BTZ but sensitive to DSF/Cu(2+). To understand the mechanism of BTZ resistance, BTZ resistant (CMY-BR) and (CMK-BR) were generated. A novel mutation PSMB5 Q62P underlied BTZ resistance, and was associated with an overexpression of the beta5 proteasome subunit. BTZ-resistance conferred increased resistance to Ara-C due to G1 arrest in the CMY-BR cells, which protected the cells from S-phase damage by Ara-C. CMY-BR and CMK-BR cells were cross-resistant to CFZ and MG-132 but sensitive to DSF/Cu(2+). In this setting, DSF/Cu(2+) induced apoptosis and proteasome inhibition independent of CT-like activity inhibition. CONCLUSIONS: We provide evidence that DSF/Cu(2+) overcomes Ara-C and BTZ resistance in cell lines from DS-AMKL patients. A novel mutation underlying BTZ resistance was detected that may identify BTZ-resistant patients, who may not benefit from treatment with CFZ or Ara-C, but may be responsive to DSF/Cu(2+). Our findings support the clinical development of DSF/Cu(2+) as a less toxic efficacious treatment approach in patients with relapsed/refractory DS-AMKL.
Subacute alcohol and/or disulfiram intake affects bioelements and redox status in rat testes.[Pubmed:28344087]
Food Chem Toxicol. 2017 Jul;105:44-51.
The aim of the study was to investigate if alcohol and Disulfiram (DSF) individually and in combination affect bioelements' and red-ox homeostasis in testes of the exposed rats. The animals were divided into groups according to the duration of treatments (21 and/or 42 days): C21/C42 groups (controls); OL21 and OL22-42 groups (0.5 mL olive oil intake); A1-21 groups (3 mL 20% ethanol intake); DSF1-21 groups (178.5 mg DSF/kg/day intake); and A21+DSF22-42 groups (the DSF ingestion followed previous 21 days' treatment with alcohol). The measured parameters in testes included metals: zinc (Zn), copper (Cu), iron (Fe), magnesium (Mg) and selenium (Se); as well as oxidative stress (OS) parameters: superoxide anion radical (O2(*-)), glutathione reduced (GSH) and oxidized (GSSG), malondialdehyde (MDA), hydrogen peroxide (H2O2) decomposition and activities of total superoxide dismutase (tSOD), glutathione-S-transferase (GST) and glutathione reductase (GR). Metal status was changed in all experimental groups (Fe rose, Zn fell, while Cu increased in A21+DSF24-32 groups). Development of OS was demonstrated in A1-21 groups, but not in DSF1-21 groups. In A21+DSF22-42 groups, OS was partially reduced compared to A groups (A1-21>MDA>C; A1-21 Alcohol consumption leads to the production of the highly reactive ethanol metabolite, acetaldehyde, which may affect intestinal tight junctions and increase paracellular permeability. We examined the effects of elevated acetaldehyde within the gastrointestinal tract on the permeability and bioavailability of hydrophilic markers and drug molecules of variable molecular weight and geometry. In vitro permeability was measured unidirectionally in Caco-2 and MDCKII cell models in the presence of acetaldehyde, ethanol, or Disulfiram, an aldehyde dehydrogenase inhibitor, which causes acetaldehyde formation when coadministered with ethanol in vivo. Acetaldehyde significantly lowered transepithelial resistance in cell monolayers and increased permeability of the low-molecular-weight markers, mannitol and sucrose; however, permeability of high-molecular-weight markers, polyethylene glycol and inulin, was not affected. In vivo permeability was assessed in male Sprague-Dawley rats treated for 6 days with ethanol, Disulfiram, or saline alone or in combination. Bioavailability of naproxen was not affected by any treatment, whereas that of paclitaxel was increased upon acetaldehyde exposure. Although Disulfiram has been shown to inhibit multidrug resistance-1 P-glycoprotein (P-gp) in vitro, our data demonstrate that the known P-gp substrate paclitaxel is not affected by coadministration of Disulfiram. In conclusion, we demonstrate that acetaldehyde significantly modulates tight junctions and paracellular permeability in vitro as well as the oral bioavailability of low-molecular-weight hydrophilic probes and therapeutic molecules in vivo even when these molecules are substrates for efflux transporters. These studies emphasize the significance of ethanol metabolism and drug interactions outside of the liver. Fluorescence intensity of the pH-sensitive carboxyfluorescein derivative 2,7-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) was monitored by high-throughput flow cytometry in living yeast cells. We measured fluorescence intensity of BCECF trapped in yeast vacuoles, acidic compartments equivalent to lysosomes where vacuolar proton-translocating ATPases (V-ATPases) are abundant. Because V-ATPases maintain a low pH in the vacuolar lumen, V-ATPase inhibition by concanamycin A alkalinized the vacuole and increased BCECF fluorescence. Likewise, V-ATPase-deficient mutant cells had greater fluorescence intensity than wild-type cells. Thus, we detected an increase of fluorescence intensity after short- and long-term inhibition of V-ATPase function. We used yeast cells loaded with BCECF to screen a small chemical library of structurally diverse compounds to identify V-ATPase inhibitors. One compound, Disulfiram, enhanced BCECF fluorescence intensity (although to a degree beyond that anticipated for pH changes alone in the mutant cells). Once confirmed by dose-response assays (EC(50)=26 microM), we verified V-ATPase inhibition by Disulfiram in secondary assays that measured ATP hydrolysis in vacuolar membranes. The inhibitory action of Disulfiram against V-ATPase pumps revealed a novel effect previously unknown for this compound. Because V-ATPases are highly conserved, new inhibitors identified could be used as research and therapeutic tools in cancer, viral infections, and other diseases where V-ATPases are involved. Cancer cells, characterized by local invasion and distant metastasis, are very much dependent on the extracellular matrix. The expression of matrix metalloproteinases (MMPs) has been implicated in the invasion and metastasis of cancer cells. In this study, we reported the effects of Disulfiram, a clinically used anti-alcoholism drug, on tumor invasion suppression, as well as its effects on the activity of MMP-2 and MMP-9 in human osteosarcoma cells (U2OS). Disulfiram has been used for alcohol aversion therapy. However, recent reports have shown that Disulfiram may have potential in the treatment of human cancers. Herewith, we showed that the anti-tumor effects of Disulfiram, in an invasion assay using U2OS cells and that Disulfiram has a type IV collagenase inhibitory activity that inhibits expression of genes and proteins responsible for both cell and non-cell mediated invasion on pathways. In conclusion, Disulfiram inhibited expression of MMP-2 and MMP-9 and it regulated the invasion of human osteosarcoma cells. These observations raise the possibility of Disulfiram being used clinical for the inhibition of cancer invasion. Disulfiram [bis(diethylthiocarbamoyl)disulphide] has been found to stimulate reversibly the Ca(2+)-ATPase of skeletal muscle sarcoplasmic reticulum. At pH 7.2, 2.1 mM ATP and 25 degrees C, ATPase activity was found to double on addition of 120 microM Disulfiram. Stimulation fitted to binding of Disulfiram at a single site with a Kd of 61 microM. Disulfiram had no effect on the Ca2+ affinity of the ATPase or on the rate of phosphorylation of the ATPase by ATP, but increased the rate of dissociation of Ca2+ from the phosphorylated ATPase (the transport step) and increased the rate of dephosphorylation of the phosphorylated ATPase. It also decreased the level of phosphorylation of the ATPase by Pi, consistent with a 7.5-fold decrease in the equilibrium constant of the phosphorylated to non-phosphorylated forms (E2PMg/E2PiMg) at 80 microM Disulfiram. Disulfiram had no significant effect on the concentration of ATP resulting in stimulation of ATPase activity, suggesting that it does not bind to the empty nucleotide-binding site on the phosphorylated ATPase. Studies of the effects of mixtures of Disulfiram and jasmone (another molecule that stimulates the ATPase) suggest that they bind to separate sites on the ATPase.The ethanol metabolite acetaldehyde increases paracellular drug permeability in vitro and oral bioavailability in vivo.[Pubmed:19820208]
J Pharmacol Exp Ther. 2010 Jan;332(1):326-33.
Identification of inhibitors of vacuolar proton-translocating ATPase pumps in yeast by high-throughput screening flow cytometry.[Pubmed:20018164]
Anal Biochem. 2010 Mar 15;398(2):203-11.
Disulfiram suppresses invasive ability of osteosarcoma cells via the inhibition of MMP-2 and MMP-9 expression.[Pubmed:18047805]
J Biochem Mol Biol. 2007 Nov 30;40(6):1069-76.
Stimulation of the Ca(2+)-ATPase of sarcoplasmic reticulum by disulfiram.[Pubmed:8947473]
Biochem J. 1996 Nov 15;320 ( Pt 1):101-5.


