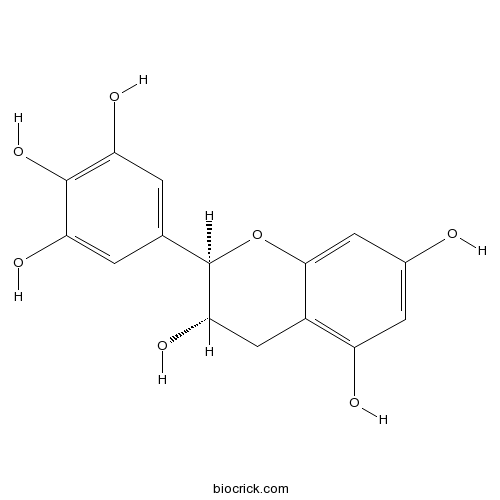
(+)-GallocatechinCAS# 970-73-0 |
- (-)-Gallocatechin
Catalog No.:BCN5927
CAS No.:3371-27-5
- (-)-Epigallocatechin(EGC)
Catalog No.:BCN4519
CAS No.:970-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 970-73-0 | SDF | Download SDF |
| PubChem ID | 65084 | Appearance | White powder |
| Formula | C15H14O7 | M.Wt | 306.27 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | ||
| SMILES | C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C(=C3)O)O)O)O | ||
| Standard InChIKey | XMOCLSLCDHWDHP-SWLSCSKDSA-N | ||
| Standard InChI | InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (+)-Gallocatechin as a bio-antimutagenic compound against UV-induced mutation in Escherichia coli. It is potent in scavenging Fremy’s salt, a synthetic free radical, it possesses antioxidant capacities that is higher or comparable to that of ascorbic acid or Trolox. |
| Targets | BACE |
| Structure Identification | Phytochemistry. 1994 Jul;36(4):1027-9.Identification of (+)-gallocatechin as a bio-antimutagenic compound in Psidium guava leaves.[Pubmed: 7765204]From the MeOH-extract of guava leaves, (+)-Gallocatechin was isolated as a bio-antimutagenic compound against UV-induced mutation in Escherichia coli. This strengthens the evidence that phenolic compounds require three neighbouring-OH groups in order to possess this activity. Eur Food Res. Technol., 2004, 219(6):605-13.Antioxidant gallocatechins, dimeric and trimeric proanthocyanidins from sea buckthorn (Hippophaë rhamnoides) pomace[Reference: WebLink]Residues such as peels and seeds that result from fruit juice production may contain substantial amounts of valuable natural antioxidants.
|

(+)-Gallocatechin Dilution Calculator

(+)-Gallocatechin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2651 mL | 16.3255 mL | 32.6509 mL | 65.3019 mL | 81.6273 mL |
| 5 mM | 0.653 mL | 3.2651 mL | 6.5302 mL | 13.0604 mL | 16.3255 mL |
| 10 mM | 0.3265 mL | 1.6325 mL | 3.2651 mL | 6.5302 mL | 8.1627 mL |
| 50 mM | 0.0653 mL | 0.3265 mL | 0.653 mL | 1.306 mL | 1.6325 mL |
| 100 mM | 0.0327 mL | 0.1633 mL | 0.3265 mL | 0.653 mL | 0.8163 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Disulfiram
Catalog No.:BCC2098
CAS No.:97-77-8
- Ac-Ala-OH
Catalog No.:BCC3189
CAS No.:97-69-8
- 2-Methylvaleric acid
Catalog No.:BCN8498
CAS No.:97-61-0
- Allantoin
Catalog No.:BCN4527
CAS No.:97-59-6
- Solvent Yellow 3
Catalog No.:BCC9150
CAS No.:97-56-3
- Isoeugenol
Catalog No.:BCN8312
CAS No.:97-54-1
- Eugenol
Catalog No.:BCN5964
CAS No.:97-53-0
- DTG
Catalog No.:BCC6812
CAS No.:97-39-2
- Momordin Ic
Catalog No.:BCN1216
CAS No.:96990-18-0
- Cisatracurium Besylate
Catalog No.:BCC4345
CAS No.:96946-42-8
- Artanin
Catalog No.:BCN4517
CAS No.:96917-26-9
- Cyproheptadine hydrochloride
Catalog No.:BCC5161
CAS No.:969-33-5
- (-)-Epigallocatechin(EGC)
Catalog No.:BCN4519
CAS No.:970-74-1
- Porfimer Sodium
Catalog No.:BCC5353
CAS No.:97067-70-4
- AMN 082 dihydrochloride
Catalog No.:BCC7344
CAS No.:97075-46-2
- 6-Geranylnaringenin
Catalog No.:BCN3001
CAS No.:97126-57-3
- 6-Epi-8-O-acetylharpagide
Catalog No.:BCN4550
CAS No.:97169-44-3
- 3-Ethoxyandrosta-3,5-dien-17-one
Catalog No.:BCC8630
CAS No.:972-46-3
- Meisoindigo
Catalog No.:BCC5132
CAS No.:97207-47-1
- Eriobofuran
Catalog No.:BCN7436
CAS No.:97218-06-9
- Picfeltarraenin IB
Catalog No.:BCN2845
CAS No.:97230-46-1
- Picfeltarraenin IA
Catalog No.:BCN1041
CAS No.:97230-47-2
- Topiramate
Catalog No.:BCC2314
CAS No.:97240-79-4
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
Effect of Stereochemical Configuration on the Transport and Metabolism of Catechins from Green Tea across Caco-2 Monolayers.[Pubmed:30917581]
Molecules. 2019 Mar 26;24(6). pii: molecules24061185.
The transcellular transport and metabolism of eight green tea catechins (GTCs) were studied in Caco-2 monolayers, with the aim of investigating the effect of cis(-)trans isomerism on the membrane permeability and biotransformation of GTCs. The results showed that the catechin stereochemistry significantly affects the efflux transport rather than the absorption transport in the Caco-2 monolayers. The trans catechins showed a better transcellular permeability than their corresponding cis (epi) catechins in the efflux transport, as the efflux amount of trans catechins were all significantly higher than that of the cis (epi) catechins at each concentration and each time point tested. Moreover, the relative contents of the (+)-catechin (C)-O-sulfate, (+)-Gallocatechin (GC)-O-sulfate, (-)-catechin gallate (CG)-O-sulfate, and (-)-gallocatechin gallate (GCG)-O-sulfate in the efflux transport were 2.67, 16.08, 50.48, and 31.54 times higher than that of the (-)-epicatechin (EC)-O-sulfate, (-)-epigallocatechin (EGC)-O-sulfate, (-)-epicatechin gallate (ECG)-O-sulfate, and (-)-epigallocatechin gallate (EGCG)-O-sulfate, respectively. It indicated that more metabolites were observed after the transcellular efflux of trans catechins. Furthermore, after two hours of incubation, the GTCs could significantly increase the expression of multidrug resistance-associated protein 2 (MRP2) and breast cancer-resistance protein (BCRP), and decrease the expression of P-glycoprotein in the Caco-2 cells. The regulation of GTCs on P-glycoprotein, MRP2, and BCRP could also be significantly influenced by the chemical and dimensional structure. In a conclusion, catechin stereochemistry significantly affects the transport and metabolism of GTCs when refluxed in the Caco-2 monolayers.
Studies on the bioactive flavonoids isolated from Azadirachta indica.[Pubmed:30835540]
Nat Prod Res. 2019 Mar 5:1-9.
Two novel natural metabolites, 3-O-butyl-(-)-epicatechin (1) and 3-O-butyl-(-)-epigallocatechin (2), as well as several known substances, (-)-epicatechin (3), (+)-Gallocatechin (4), (-)-epigallocatechin (5), azadirachtin A (6), trilinolein (7) and octadecanoic acid-tetrahydrofuran-3,4-diyl ester (8), were isolated from the bark of Azadirachta indica. The structures of all compounds were established by comprehensive and comparative spectroscopic analysis of NMR and ESI-HRMS data. The new metabolites 1 and 2 represent one of the few examples of natural compounds with a butyl ether group moiety. The acaricidal activity of the compounds was tested using a standard Shaw larval immersion assay. All the compounds, except 7, possess a LD50 value less than or equal to 7.2 mM.


