DTGCAS# 97-39-2 |
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 97-39-2 | SDF | Download SDF |
| PubChem ID | 7333 | Appearance | Powder |
| Formula | C15H17N3 | M.Wt | 239.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 150 mg/mL (626.78 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
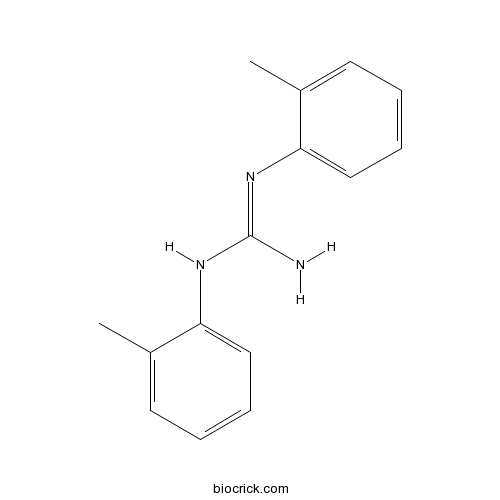
| Chemical Name | 1,2-bis(2-methylphenyl)guanidine | ||
| SMILES | CC1=CC=CC=C1NC(=NC2=CC=CC=C2C)N | ||
| Standard InChIKey | OPNUROKCUBTKLF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H17N3/c1-11-7-3-5-9-13(11)17-15(16)18-14-10-6-4-8-12(14)2/h3-10H,1-2H3,(H3,16,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Displays high and roughly equal affinity for both σ1 and σ2 receptors. |

DTG Dilution Calculator

DTG Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1785 mL | 20.8925 mL | 41.7851 mL | 83.5701 mL | 104.4626 mL |
| 5 mM | 0.8357 mL | 4.1785 mL | 8.357 mL | 16.714 mL | 20.8925 mL |
| 10 mM | 0.4179 mL | 2.0893 mL | 4.1785 mL | 8.357 mL | 10.4463 mL |
| 50 mM | 0.0836 mL | 0.4179 mL | 0.8357 mL | 1.6714 mL | 2.0893 mL |
| 100 mM | 0.0418 mL | 0.2089 mL | 0.4179 mL | 0.8357 mL | 1.0446 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Momordin Ic
Catalog No.:BCN1216
CAS No.:96990-18-0
- Cisatracurium Besylate
Catalog No.:BCC4345
CAS No.:96946-42-8
- Artanin
Catalog No.:BCN4517
CAS No.:96917-26-9
- Cyproheptadine hydrochloride
Catalog No.:BCC5161
CAS No.:969-33-5
- 1,2-Bis(4'-methyl-2,2'-bipyridin-4-yl)ethane
Catalog No.:BCC8414
CAS No.:96897-04-0
- Nyasol
Catalog No.:BCN7579
CAS No.:96895-25-9
- VIP (guinea pig)
Catalog No.:BCC5725
CAS No.:96886-24-7
- XAC
Catalog No.:BCC7600
CAS No.:96865-92-8
- XCC
Catalog No.:BCC7890
CAS No.:96865-83-7
- Maoecrystal A
Catalog No.:BCN5407
CAS No.:96850-30-5
- Maoecrystal B
Catalog No.:BCN4516
CAS No.:96850-29-2
- 8beta-Tigloyloxycostunolide
Catalog No.:BCN7115
CAS No.:96850-21-4
- Eugenol
Catalog No.:BCN5964
CAS No.:97-53-0
- Isoeugenol
Catalog No.:BCN8312
CAS No.:97-54-1
- Solvent Yellow 3
Catalog No.:BCC9150
CAS No.:97-56-3
- Allantoin
Catalog No.:BCN4527
CAS No.:97-59-6
- 2-Methylvaleric acid
Catalog No.:BCN8498
CAS No.:97-61-0
- Ac-Ala-OH
Catalog No.:BCC3189
CAS No.:97-69-8
- Disulfiram
Catalog No.:BCC2098
CAS No.:97-77-8
- (+)-Gallocatechin
Catalog No.:BCN5928
CAS No.:970-73-0
- (-)-Epigallocatechin(EGC)
Catalog No.:BCN4519
CAS No.:970-74-1
- Porfimer Sodium
Catalog No.:BCC5353
CAS No.:97067-70-4
- AMN 082 dihydrochloride
Catalog No.:BCC7344
CAS No.:97075-46-2
- 6-Geranylnaringenin
Catalog No.:BCN3001
CAS No.:97126-57-3
Co-pyrolysis characteristics of municipal sewage sludge and hazelnut shell by TG-DTG-MS and residue analysis.[Pubmed:28236506]
Waste Manag. 2017 Apr;62:91-100.
Co-pyrolysis characteristics of municipal sewage sludge and hazelnut shell blend have been studied in this work. The behavior of co-pyrolysis was researched by a method of multi-heating rates and different blend ratios to analyze thermal decomposition stages. The experimental data of the blended samples in TG-DTG plots were compared with calculated data to investigate the interactions during co-pyrolysis. The bio-chars investigated by SEM and FTIR spectra were used to examine the physical and chemical changes. The results showed there are four thermal decomposition stages during co-pyrolysis, with hydrocarbon transforming to gas evolution in the second and the third stages. The inhibitive interaction occurred between 260 and 400 degrees C and the accelerative interaction occurred between 450 and 900 degrees C during co-pyrolysis. The activation energy of the blended sample was 51.97-178.84kJ/mol in the second stage and 207.04-630.73kJ/mol in the third stage calculated by DAEM.
Effect on HIV-1 viral replication capacity of DTG-resistance mutations in NRTI/NNRTI resistant viruses.[Pubmed:27130466]
Retrovirology. 2016 Apr 30;13(1):31.
BACKGROUND: Recommended regimens for HIV-positive individuals include the co-administration of dolutegravir (DTG) with two reverse transcriptase inhibitors (RTIs). Although rare, emerging resistance against DTG is often associated with the R263K substitution in integrase. In-vitro-selected R263K was associated with impaired viral replication capacity, DNA integration, and integrase strand-transfer activity, especially when accompanied by the secondary mutation H51Y. Given the reduced fitness of RTI-resistant viruses, we investigated potential impacts on viral replication of combining R263K and H51Y/R263K with major RTI-resistance substitutions including K65R, L74V, K103N, E138K, and M184I/V. RESULTS: We combined the R263K or H51Y/R263K with RTI-resistance mutations into the proviral plasmid pNL4.3 and measured the resulting viral infectiousness, replication capacity, and ability to integrate viral DNA into host cells. Infectiousness was determined by luciferase assay in TZM-bl cells. Replicative capacity was monitored over 7 days and viral DNA integration was studied by real-time Alu-qPCR in PM1 cells. We found that viral infectiousness, replication capacities and integration levels were greatly reduced in triple mutants, i.e. H51Y/R263K plus a RT mutation, and moderately reduced in double mutants, i.e. R263K plus a RT mutation, compared to wild-type and single RT-mutant viruses. CONCLUSIONS: Our findings help to explain the absence of RTI mutations in individuals who experienced DTG-treatment failure.
Dolutegravir(DTG, S/GSK1349572) combined with other ARTs is superior to RAL- or EFV-based regimens for treatment of HIV-1 infection: a meta-analysis of randomized controlled trials.[Pubmed:27617024]
AIDS Res Ther. 2016 Sep 8;13(1):30.
BACKGROUND: The first-generation integrase inhibitors (INIs) raltegravir (RAL) and elvitegravir (EVG) have shown efficacy against HIV infection, but they have the limitations of once-more daily dosing and extensive cross-resistance. Dolutegravir (DTG, S/GSK1349572), a second-generation drug that overcomes such shortcomings, is under spotlight. The purpose of this study is to review the evidence for DTG use in clinical settings, including its efficacy and safety. METHODS: PubMed, EMbase, Ovid, Web of Science, Science Direct, and related websites were screened from establishment until July 2013, and scientific meeting proceedings were manually searched. Two reviewers independently screened 118 citations repeatedly to identify randomized controlled trials comparing the efficacy and safety of DTG-based regimen with those of RAL- or elvitegravir-based regimens. Using the selected studies with comparable outcome measures and indications, we performed a meta-analysis based on modified intention-to-treat (mITT), on-treatment (OT), and as-treated (AT) virological outcome data. Independent data extraction and quality assessment were conducted. RESULTS: Four unique studies were included with the use of DTG in antiretroviral therapy-naive patients. In therapy-naive patients, DTG combined with abacavir/lamivudine (ABC/3TC) or tenofovir/emtricitabine (TDF/FTC) resulted in a significantly better virological outcome with a mITT relative risk (RR)of 1.07 (95 % confidence interval (95 % CI 1.03-1.12). Evidence further supported use of DTG had a better virological suppression in the 50 mg once daily group (mITT RR 1.07; 95 % CI 1.03-1.12) as well as in the sub-analysis in dolutegravir/efavirenz(DTG/EFV) and dolutegravir/raltegravir (DTG/RAL) groups (RR 1.09, 95 % CI 1.03-1.15; RR 1.06, 95 % CI 0.98-1.15, respectively). In the matter of safety of DTG-based regimen, the risk of any event was RR 0.98 (95 % CI 0.94-1.01), the risk of serious adverse events (AEs) was RR 0.84 (95 % CI 0.62-1.15), and the risk of drug-related serious AEs was RR 0.33 (95 % CI 0.13-0.79). CONCLUSION: In general, DTG 50 mg given once daily combined with an active background drug is a better choice in terms of both efficacy and safety.
Synthesis and binding characteristics of potential SPECT imaging agents for sigma-1 and sigma-2 binding sites.[Pubmed:8496936]
J Med Chem. 1993 Mar 5;36(5):566-71.
2-, 3-, and 4-idophenyl derivatives of the high-affinity sigma ligand N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine (1) were synthesized in two to four steps starting from N-methyl-2-(1-pyrrolidinyl)ethylamine. These compounds were evaluated for their capacity to label both sigma 1 and sigma 2 subtypes in vitro. sigma-1 binding affinity was determined by measuring competition with [3H]-(+)-pentazocine binding to guinea pig brain membranes while sigma 2 binding was evaluated through competition with [3H]DTG binding to rat liver membranes in the presence of excess dextrallorphan. The binding data revealed that N-[2-(3-iodophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine (2) and N-[2-(4-iodophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine (3) displayed almost identical binding affinity at sigma 1 sites to the parent compound 1. This suggests that the 3- or 4-iodo group can effectively substitute for the 3,4-dichloro substituents of 1. In this series of compounds, Ki's at the sigma 1 site varied from 2.0 nM for N-(4-iodobenzyl)-N-methyl-2-(1-pyrrolidinyl)ethylamine (6) to 26.6 nM for N-(2-iodobenzyl)-N-methyl-2-(1-pyrrolidinyl)ethylamine (4). Ki's for sigma 2 site ranged from 8.1 nM for 1 to 220 nM for N-(3-bromobenzyl)-N-methyl-2-(1-pyrrolidinyl)ethylamine (11) while the sigma 2/sigma 1 ratio varied from 1.8 for 4 to 25 for 11. Comparing halogen substitution, the trend Cl = I > Br > F was observed for binding affinity at sigma 1 sites; no such trend was observed at sigma 2 sites. On the basis of the binding data, compounds 2 and 3 were selected for labeling with 123I. Thus, treatment of the corresponding 3- and 4-(tributylstannyl) intermediates (7 and 8) with Na123I in the presence of excess CH3CO3H furnished [123I]-2 and [123I]-3 in up to 70% radiochemical yield. Preliminary in vitro binding with [123I]-3 indicated up to 97% specific binding with guinea pig brain membranes.


