NyasolCAS# 96895-25-9 |
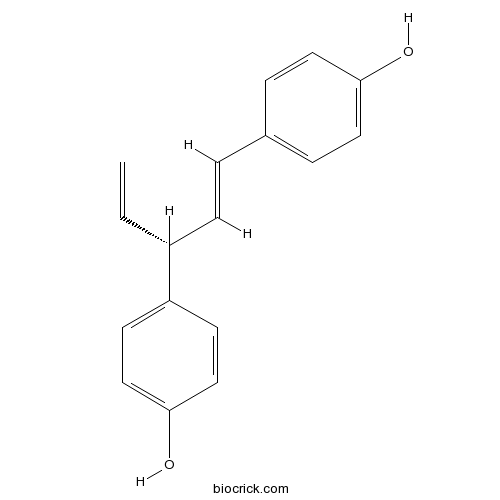
- trans-Hinokiresinol
Catalog No.:BCN1130
CAS No.:17676-24-3
- (+)-Nyasol
Catalog No.:BCN9225
CAS No.:185020-38-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 96895-25-9 | SDF | Download SDF |
| PubChem ID | 6438674 | Appearance | Oil |
| Formula | C17H16O2 | M.Wt | 252.31 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(1E,3R)-3-(4-hydroxyphenyl)penta-1,4-dienyl]phenol | ||
| SMILES | C=CC(C=CC1=CC=C(C=C1)O)C2=CC=C(C=C2)O | ||
| Standard InChIKey | VEAUNWQYYMXIRB-BOTMBNHJSA-N | ||
| Standard InChI | InChI=1S/C17H16O2/c1-2-14(15-7-11-17(19)12-8-15)6-3-13-4-9-16(18)10-5-13/h2-12,14,18-19H,1H2/b6-3+/t14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (-)-Nyasol exhibits the half maximal inhibitory concentration (IC50) value of 17.6 uM against D-glycero-α-D-manno-heptose-1-phosphate guanylyltransferase (HddC). 2. Nyasol may have potential to be developed as medicines for the treatment of allergies by inhibiting the activation of mast cells. 3. Nyasol shows anti-inflammatory properties by inhibiting iNOS expression. 4. Nyasol has antifungal activity, it is significantly effective in suppressing the Phytophthora blight on pepper plants. 5. (+)-Nyasol has antiprotozoal activity, it potently inhibits the growth of Leishmania major promastigotes, the IC50 being 12 microM, and moderately inhibits Plasmodium falciparum schizonts with the IC50 49 microM. |
| Targets | NOS | NO | IL Receptor | IFN-γ | NF-kB | Akt | ERK | IkB | Antifection | IKK |

Nyasol Dilution Calculator

Nyasol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9634 mL | 19.8169 mL | 39.6338 mL | 79.2676 mL | 99.0845 mL |
| 5 mM | 0.7927 mL | 3.9634 mL | 7.9268 mL | 15.8535 mL | 19.8169 mL |
| 10 mM | 0.3963 mL | 1.9817 mL | 3.9634 mL | 7.9268 mL | 9.9084 mL |
| 50 mM | 0.0793 mL | 0.3963 mL | 0.7927 mL | 1.5854 mL | 1.9817 mL |
| 100 mM | 0.0396 mL | 0.1982 mL | 0.3963 mL | 0.7927 mL | 0.9908 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- VIP (guinea pig)
Catalog No.:BCC5725
CAS No.:96886-24-7
- XAC
Catalog No.:BCC7600
CAS No.:96865-92-8
- XCC
Catalog No.:BCC7890
CAS No.:96865-83-7
- Maoecrystal A
Catalog No.:BCN5407
CAS No.:96850-30-5
- Maoecrystal B
Catalog No.:BCN4516
CAS No.:96850-29-2
- 8beta-Tigloyloxycostunolide
Catalog No.:BCN7115
CAS No.:96850-21-4
- Indatraline hydrochloride
Catalog No.:BCC7123
CAS No.:96850-13-4
- Przewaquinone C
Catalog No.:BCN3003
CAS No.:96839-29-1
- Orlistat
Catalog No.:BCC3830
CAS No.:96829-58-2
- Chlorovaltrate K
Catalog No.:BCN7126
CAS No.:96801-92-2
- (±)-McN 5652
Catalog No.:BCC7267
CAS No.:96795-89-0
- Chlorisondamine diiodide
Catalog No.:BCC6885
CAS No.:96750-66-2
- 1,2-Bis(4'-methyl-2,2'-bipyridin-4-yl)ethane
Catalog No.:BCC8414
CAS No.:96897-04-0
- Cyproheptadine hydrochloride
Catalog No.:BCC5161
CAS No.:969-33-5
- Artanin
Catalog No.:BCN4517
CAS No.:96917-26-9
- Cisatracurium Besylate
Catalog No.:BCC4345
CAS No.:96946-42-8
- Momordin Ic
Catalog No.:BCN1216
CAS No.:96990-18-0
- DTG
Catalog No.:BCC6812
CAS No.:97-39-2
- Eugenol
Catalog No.:BCN5964
CAS No.:97-53-0
- Isoeugenol
Catalog No.:BCN8312
CAS No.:97-54-1
- Solvent Yellow 3
Catalog No.:BCC9150
CAS No.:97-56-3
- Allantoin
Catalog No.:BCN4527
CAS No.:97-59-6
- 2-Methylvaleric acid
Catalog No.:BCN8498
CAS No.:97-61-0
- Ac-Ala-OH
Catalog No.:BCC3189
CAS No.:97-69-8
General assay for enzymes in the heptose biosynthesis pathways using electrospray ionization mass spectrometry.[Pubmed:28280867]
Appl Microbiol Biotechnol. 2017 Jun;101(11):4521-4532.
The ADP-L-glycero-beta-D-manno-heptose and the GDP-6-deoxy-alpha-D-manno-heptose biosynthesis pathways play important roles in constructing lipopolysaccharide of Gram-negative bacteria. Blocking the pathways is lethal or increases antibiotic susceptibility to pathogens. Therefore, the enzymes involved in the pathways are novel antibiotic drug targets. Here, we designed an efficient method to assay the whole enzymes in the pathways using mass spectrometry and screened 148 compounds. One promising lead is (-)-Nyasol targeting D-glycero-alpha-D-manno-heptose-1-phosphate guanylyltransferase (HddC) included in the GDP-6-deoxy-alpha-D-manno-heptose biosynthesis pathway from Burkholderia pseudomallei. The inhibitory activity of the lead compound against HddC has been confirmed by blocking the system transferring the guanosine monophosphate (GMP) moiety to alpha-D-glucose-1-phosphate. (-)-Nyasol exhibits the half maximal inhibitory concentration (IC50) value of 17.6 muM. A further study is going on using (-)-Nyasol derivatives to find better leads with high affinity.
Isolation and anti-oomycete activity of nyasol from Anemarrhena asphodeloides rhizomes.[Pubmed:14561517]
Phytochemistry. 2003 Nov;64(5):997-1001.
The methanol extract of Anemarrhena asphodeloides rhizomes exhibited strong antifungal activity against the plant pathogenic fungi Magnaphothe grisea, Rhizoctonia solani, and the plant pathogenic oomycete Phytophthora capsici. The antifungal substance isolated from the rhizomes of A. asphodeloides was identified to be Nyasol, (Z)-1,3-bis(4-hydroxyphenyl)-1,4-pentadiene by NMR and mass spectral analysis. Nyasol effectively inhibited the mycelial growth of Colletotrichum orbiculare, P. capsici, Pythium ultimum, R. solani, and Cladosporium cucumerinum in a range of 1-50 mug/ml, but did not affect the growth of bacteria and yeast. In a greenhouse test, treatment with the antifungal compound Nyasol was significantly effective in suppressing the Phytophthora blight on pepper plants.
Suppression of inducible nitric oxide synthase expression by nyasol and broussonin A, two phenolic compounds from Anemarrhena asphodeloides, through NF-kappaB transcriptional regulation in vitro and in vivo.[Pubmed:24827684]
Chem Biodivers. 2014 May;11(5):749-59.
Anemarrhena asphodeloides is widely used in traditional Chinese medicine, and is known to possess antidiabetic and anti-inflammatory properties. Because inducible nitric oxide synthase (iNOS) plays an important role in inflammation, we investigated the inhibitory effects of two known phenolic compounds, Nyasol (1) and broussonin A (2), from A. asphodeloides, on iNOS and its plausible mechanism of action. Compounds 1 and 2 exhibited inhibitory effects on nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. Compounds 1 and 2 also suppressed the expressions of iNOS protein and mRNA. Moreover, compounds 1 and 2 suppressed the expression of inflammatory cytokines such as interleukin-1beta (IL-1beta) and interferon-beta (IFN-beta). They also inhibited the transcriptional activity of NF-kappaB and degradation of IkappaB-alpha, as well as the activation of Akt and ERK in LPS-stimulated RAW 264.7 cells. In in vivo animal model, compounds 1 and 2 significantly inhibited TPA-induced mouse ear edema. These results suggest that 1 and 2 suppress LPS-stimulated iNOS expression at the transcriptional level through modulating NF-kappaB and down-regulation of the Akt and ERK signaling pathways. Taken together, these findings indicate that the suppressive effects of 1 and 2 on iNOS expression might provide one possible mechanism for their anti-inflammatory activities.
Antiprotozoal compounds from Asparagus africanus.[Pubmed:9358645]
J Nat Prod. 1997 Oct;60(10):1017-22.
Two antiprotozoal compounds have been isolated from the roots of Asparagus africanus Lam. (Liliaceae), a new sapogenin, 2 beta, 12 alpha-dihydroxy-(25R)-spirosta-4,7-dien-3-one (1), which was named muzanzagenin, and the lignan (+)-Nyasol (2), (Z)-(+)-4,4'-(3-ethenyl-1-propene-1,3-diyl)-bisphenol. The structure of the sapogenin was elucidated by MS and by 1D and 2D NMR methods and established by a single crystal X-ray analysis. (+)-Nyasol potently inhibits the growth of Leishmania major promastigotes, the IC50 being 12 microM, and moderately inhibits Plasmodium falciparum schizonts with the IC50 49 microM. These concentrations only moderately affect the proliferation of human lymphocytes. Muzanzagenin showed a moderate in vitro activity in all three tests, the IC50 against leishmania promastigotes was 70 microM, and against four different malaria schizont strains the IC50 values were 16, 163, 23, and 16 microM, respectively.
Inhibitory effects of norlignans isolated from Anemarrhena asphodeloides on degranulation of rat basophilic leukemia- 2H3Cells.[Pubmed:27780134]
Biomed Pharmacother. 2016 Dec;84:1061-1066.
Anemarrhena asphodeloides is known to suppress inflammation and lower various fevers. To determine the active component of A. asphodeloides, ethanol (EtOH) extract of A. asphodeloides rhizomes was fractionized. The compounds isolated from the dichloromethane (CH2Cl2) soluble fraction were identified as 4'-O-methylNyasol (1), Nyasol (2), 3''-methoxyNyasol (3), 3''-hydroxy-4''-methoxy-4''-dehydroxyNyasol (4), 4-hydroxybenzaldehyde (5), and 4-hydroxyacetophenone (6). The four norlignans (1-4) potently inhibited the release of beta-hexosaminidase from immunoglobulin E (IgE)/dinitrophenol-conjugated bovine serum albumin (DNP-BSA)-treated rat basophilic leukemia (RBL)-2H3 and A23187 plus phorbol 12-myristate 13-acetate co-treated isolated rat primary mast cells, as markers of degranulation and histamine release. The intraperitoneal treatment with the EtOH extract significantly suppressed the fetal reaction, and serum histamine release induced by compound 48/80 in mice. These results suggest that the four active norlignan compounds and the EtOH extract of A. asphodeloides may have potential to be developed as medicines for the treatment of allergies by inhibiting the activation of mast cells.


