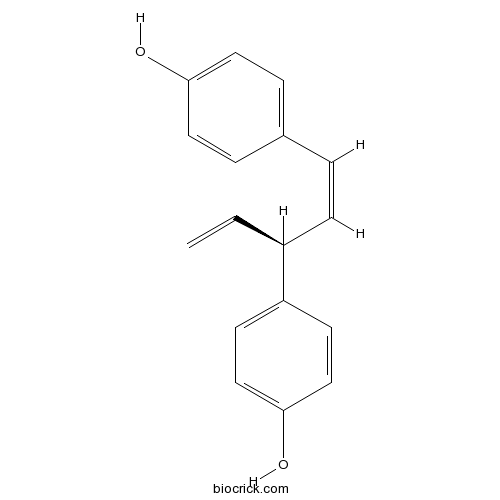
trans-HinokiresinolCAS# 17676-24-3 |
- Nyasol
Catalog No.:BCN7579
CAS No.:96895-25-9
- (+)-Nyasol
Catalog No.:BCN9225
CAS No.:185020-38-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17676-24-3 | SDF | Download SDF |
| PubChem ID | 5281830 | Appearance | Powder |
| Formula | C17H16O2 | M.Wt | 252.3 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | hinokiresinol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(1Z,3S)-3-(4-hydroxyphenyl)penta-1,4-dienyl]phenol | ||
| SMILES | C=CC(C=CC1=CC=C(C=C1)O)C2=CC=C(C=C2)O | ||
| Standard InChIKey | VEAUNWQYYMXIRB-XSHSDMCLSA-N | ||
| Standard InChI | InChI=1S/C17H16O2/c1-2-14(15-7-11-17(19)12-8-15)6-3-13-4-9-16(18)10-5-13/h2-12,14,18-19H,1H2/b6-3-/t14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Hinokiresinol (trans-hinokiresinol) and nyasol (cis-hinokiresinol) possess appreciable estrogen receptor binding activity, they can stimulate the proliferation of estrogen- dependent T47D breast cancer cells, and their stimulatory effects could be blocked by an estrogen antagonist, indicating that they are estrogen agonists. 2. trans- and cis-Hinokiresinols have similar free radical scavenging and anti-inflammatory activities, they also have anti-ischemic effects, only trans-hinokiresinol can significantly decrease neuronal injury in cultured cortical neurons exposed to oxygen-glucose deprivation followed by re-oxygenation. 3. Hinokiresinol is a novel inhibitor of LTB4 binding to the human neutrophils. 4. Hinokiresinol has antiallergic effect, it inhibits IgE-induced mouse passive cutaneous anaphylaxis reaction. |
| Targets | Estrogen receptor | NOS | IL Receptor | TNF-α | Progestogen receptor |

trans-Hinokiresinol Dilution Calculator

trans-Hinokiresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9635 mL | 19.8177 mL | 39.6354 mL | 79.2707 mL | 99.0884 mL |
| 5 mM | 0.7927 mL | 3.9635 mL | 7.9271 mL | 15.8541 mL | 19.8177 mL |
| 10 mM | 0.3964 mL | 1.9818 mL | 3.9635 mL | 7.9271 mL | 9.9088 mL |
| 50 mM | 0.0793 mL | 0.3964 mL | 0.7927 mL | 1.5854 mL | 1.9818 mL |
| 100 mM | 0.0396 mL | 0.1982 mL | 0.3964 mL | 0.7927 mL | 0.9909 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Phorbol
Catalog No.:BCN3726
CAS No.:17673-25-5
- KN-92
Catalog No.:BCC1680
CAS No.:176708-42-2
- Delphinidin-3,5-O-diglucoside chloride
Catalog No.:BCN1522
CAS No.:17670-06-3
- Ducheside A
Catalog No.:BCN1129
CAS No.:176665-78-4
- H-D-Leu-OBzl.TosOH
Catalog No.:BCC2976
CAS No.:17664-93-6
- Urocortin (human)
Catalog No.:BCC5788
CAS No.:176591-49-4
- AAL Toxin TE2
Catalog No.:BCN1742
CAS No.:176590-38-8
- AAL Toxin TE1
Catalog No.:BCN1737
CAS No.:176590-37-7
- AAL Toxin TD2
Catalog No.:BCN1740
CAS No.:176590-36-6
- AAL Toxin TD1
Catalog No.:BCN1735
CAS No.:176590-35-5
- AAL Toxin TC2
Catalog No.:BCN1741
CAS No.:176590-34-4
- AAL Toxin TC1
Catalog No.:BCN1736
CAS No.:176590-33-3
- Neoruscogenin
Catalog No.:BCN8168
CAS No.:17676-33-4
- Ageratochromene dimer
Catalog No.:BCN8110
CAS No.:17678-76-1
- (RS)-3,4-DCPG
Catalog No.:BCC7045
CAS No.:176796-64-8
- Homoplantaginin
Catalog No.:BCN2488
CAS No.:17680-84-1
- Metergoline
Catalog No.:BCC6709
CAS No.:17692-51-2
- CCT007093
Catalog No.:BCC5147
CAS No.:176957-55-4
- LY 320135
Catalog No.:BCC7346
CAS No.:176977-56-3
- 2alpha,19alpha-Dihydroxy-3-oxo-urs-12-en-28-oic acid
Catalog No.:BCN7406
CAS No.:176983-21-4
- Ambrisentan
Catalog No.:BCC4887
CAS No.:177036-94-1
- R 568 hydrochloride
Catalog No.:BCC7781
CAS No.:177172-49-5
- 26-O-Acetylsootepin A
Catalog No.:BCN7699
CAS No.:1772588-99-4
- Aglain B
Catalog No.:BCN6636
CAS No.:177262-32-7
Stereochemistry of cis- and trans-hinokiresinol and their estrogen-like activity.[Pubmed:10726863]
Chem Pharm Bull (Tokyo). 2000 Mar;48(3):389-92.
Naturally occurring phenylpropanoids, hinokiresinol (trans-Hinokiresinol) and nyasol (cis-hinokiresinol) were found to possess appreciable estrogen receptor binding activity. Strong differences in activity were observed between the geometrical isomers and enantiomers. Among these, (3S)-cis-hinokiresinol displayed the highest activity, one order of magnitude greater than the activity of genistein. Furthermore, cis- and trans-Hinokiresinol stimulated the proliferation of estrogen-dependent T47D breast cancer cells, and their stimulatory effects were blocked by an estrogen antagonist, indicating that the compounds are estrogen agonists. In addition, the absolute configuration of C-3 in (+)-cis-hinokiresinol has been assigned as S by comparison with the circular dichroism spectra of the hydrogenated products prepared from cis and trans ((3S)-trans-Hinokiresinol: previously assigned) isomers. These results incidentally provide us with an unambiguous answer to contradictory reports regarding the assignment of the full stereochemisry of cis- and trans-Hinokiresinol that have existed in the literature for more than two decades.
Effects of Chamaecyparis formosensis Matasumura extractives on lipopolysaccharide-induced release of nitric oxide.[Pubmed:17291735]
Phytomedicine. 2007 Oct;14(10):675-80.
Chamaecyparis formaosensis, commonly known as Taiwan red cypress, is native to Taiwan and grows at elevations of 1500-2150 m in Taiwan's central mountains. Many compounds have been identified from different pasts of C. formosensis, but up until now, little research has been done on the link between the constituents of C. formosensis and its bioactivities. In this study, we found that an ethyl acetate fraction (EA) of methonal extract of C. formosecsis, strongly inhibited LPS-mediated nitric oxide (NO) production in Raw 264.7 cells. The EA was further divided into 25 subfractions (EA1-EA25) by column chromatography. EA12 possessed the strongest NO production inhibition activity (IC(50) was 4.1 microg/mL). At a dosage of 20 microg/mL, EA12 completely inhibited NO production and the mRNA expression of inducible nitric oxide synthase (iNOS) in LPS-stimulated macrophage RAW264.7 cells. Bioactivity-guided chromatographic fractionation and metabolite profiling coupled with spectroscopic analyses, including (1)H-NMR, (13)C-NMR analyses, identified six compounds: vanillin (1), 4-hydroxybenzaldehyde (2), trans-Hinokiresinol (3), taiwanin E (4), 4alpha-hydroxyeudesm- 11-en-12-al (5), savinin (6). All of these six compounds were the first identified and reported from this tree species. Compounds (1), (3) and (5) demonstrated significant NO inhibition effect through reduction of NO production in activated RAW 264.7 cells due to the suppression of iNOS gene expression: compounds that can selectively inhibit undesirable expression of iNOS are important as they may serve as potential cancer chemopreventatives. This study suggests that C. formosensis may have potential for use as a natural resource for human health care.
Differential anti-ischemic efficacy and therapeutic time window of trans- and cis-hinokiresinols: stereo-specific antioxidant and anti-inflammatory activities.[Pubmed:23287539]
Neuropharmacology. 2013 Apr;67:465-75.
During cerebral ischemia, neurons are injured by various mechanisms including excitotoxicity, oxidative stress, and inflammatory responses. Thus, pharmacological manipulation of multiple cytotoxic pathways has been pursued for the treatment of ischemic injury. Cis-hinokiresinol, a naturally occurring phenylpropanoid, was previously reported to possess anti-oxidant, anti-inflammatory and estrogen-like activities. In the present study, we investigated anti-ischemic effects of trans- and cis-hinokiresinols using in vitro as well as in vivo experimental models. The ORAC and DPPH assays showed that two isomers had similar free radical scavenging activities. However, only trans-Hinokiresinol significantly decreased neuronal injury in cultured cortical neurons exposed to oxygen-glucose deprivation (75 min) followed by re-oxygenation (9 h). The differential neuroprotective effect could be due to the stereo-specific augmentation of Cu/Zn-SOD activity by trans-Hinokiresinol, when compared with cis-hinokiresinol. Similarly, in rats subjected to transient middle cerebral artery occlusion (1.5 h) followed by 24-h reperfusion, pre-ischemic treatment with trans-Hinokiresinol, but not with cis-isomer, reduced cerebral infarct volume. Interestingly, however, post-ischemic treatment with both hinokiresinols (2 and 7 h after onset of ischemia) significantly reduced cerebral infarct. When administered after onset of ischemia, trans-Hinokiresinol, but not its cis-isomer reduced nitrotyrosine immunoreactivity in ischemic regions. In contrast, both hinokiresinols suppressed neutrophil infiltration and IL-1beta release to a similar extent. The observed differential anti-oxidant, but comparable anti-inflammatory, activities may explain the stereo-specific anti-ischemic activities and different therapeutic time windows of the hinokiresinols examined. More detailed delineation of the anti-ischemic mechanism(s) of hinokiresinols may provide a better strategy for development of efficacious regimens for cerebral ischemic stroke.
Hinokiresinol inhibits IgE-induced mouse passive cutaneous anaphylaxis reaction.[Pubmed:17051467]
Planta Med. 2006 Nov;72(14):1328-30.
The antiallergic effect of hinokiresinol isolated from the whole plant of TRAPA Pseudoincisa S. at. Z. was measured in vitro and in vivo. Hinokiresinol not only potently inhibited beta-hexosaminidase release from RBL-2H3 cells induced by IgE, with an IC50 value of 98 microM, but also inhibited the proinflammatory cytokines IL-6, IL-4 and TNF-alpha in RBL-2H3 cells stimulated by IgE. Orally and intraperitoneally administered hinokiresinol potently inhibited the passive anaphylaxis reaction in mice induced by IgE.
Hinokiresinol is not a precursor of agatharesinol in the norlignan biosynthetic pathway in Japanese cedar.[Pubmed:16884819]
J Plant Physiol. 2006 Dec;163(12):1221-8.
The biosynthetic relationship between the two norlignans agatharesinol and trans-Hinokiresinol was investigated. Fresh sapwood sticks of Cryptomeria japonica were fed with stable isotope-labeled compounds, namely p-coumaryl alcohol-[9,9-(2)H], p-coumaryl alcohol-[9-(18)O] and trans-Hinokiresinol-[1-(2)H], and then incubated under high-humidity for approximately 20 days, during which the two norlignans were produced simultaneously. While trans-Hinokiresinol was strongly deuterium-labeled after feeding with p-coumaryl alcohol-[9,9-(2)H], agatharesinol was only lightly labeled after feeding with either p-coumaryl alcohol-[9,9-(2)H] or -[9-(18)O]. These results suggest that p-coumaryl alcohol, which is a precursor of hinokiresinol, is not involved in the biosynthesis of agatharesinol. Therefore, the norlignan carbon skeleton of agatharesinol must be framed from different types of phenylpropanoid monomers compared to those utilized by the trans-Hinokiresinol pathway. The biosynthesis of these two norlignans seems to branch at an early stage, i.e., before the framing of the norlignan carbon skeleton. Furthermore, agatharesinol was not labeled with deuterium after feeding with (2)H-labeled trans-Hinokiresinol, which has the simplest norlignan structure. This result strongly supports the suggestion that the conversion of trans-Hinokiresinol to agatharesinol is not part of the biosynthesis of norlignans and that early branching occurs instead.


