AmbrisentanETA-receptor antagonist CAS# 177036-94-1 |
- AM-095 free base
Catalog No.:BCC1352
CAS No.:1228690-36-5
- AM095
Catalog No.:BCC1351
CAS No.:1345614-59-6
- Ki16198
Catalog No.:BCC4560
CAS No.:355025-13-7
- Ki16425
Catalog No.:BCC1155
CAS No.:355025-24-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 177036-94-1 | SDF | Download SDF |
| PubChem ID | 197712 | Appearance | Powder |
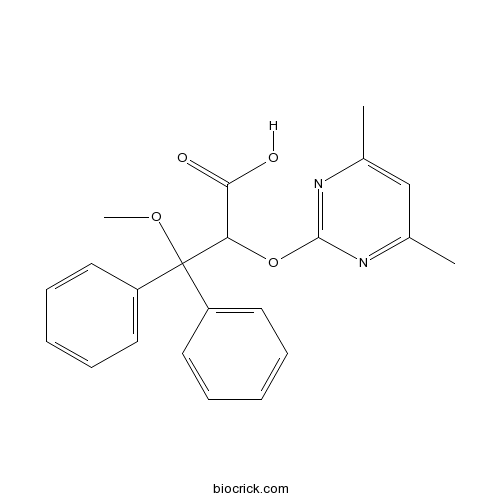
| Formula | C22H22N2O4 | M.Wt | 378.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (264.26 mM; Need ultrasonic) Ethanol : 7.14 mg/mL (18.87 mM; Need ultrasonic) | ||
| Chemical Name | 2-(4,6-dimethylpyrimidin-2-yl)oxy-3-methoxy-3,3-diphenylpropanoic acid | ||
| SMILES | CC1=CC(=NC(=N1)OC(C(=O)O)C(C2=CC=CC=C2)(C3=CC=CC=C3)OC)C | ||
| Standard InChIKey | OUJTZYPIHDYQMC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H22N2O4/c1-15-14-16(2)24-21(23-15)28-19(20(25)26)22(27-3,17-10-6-4-7-11-17)18-12-8-5-9-13-18/h4-14,19H,1-3H3,(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective endothelin receptor ETA antagonist. Displays approximately 200-fold selectivity for ETA over ETB (Ki values are 1 nM and 195 nM for respectively). Prevents endothelin-induced sudden death in rats. |

Ambrisentan Dilution Calculator

Ambrisentan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6426 mL | 13.2128 mL | 26.4257 mL | 52.8513 mL | 66.0642 mL |
| 5 mM | 0.5285 mL | 2.6426 mL | 5.2851 mL | 10.5703 mL | 13.2128 mL |
| 10 mM | 0.2643 mL | 1.3213 mL | 2.6426 mL | 5.2851 mL | 6.6064 mL |
| 50 mM | 0.0529 mL | 0.2643 mL | 0.5285 mL | 1.057 mL | 1.3213 mL |
| 100 mM | 0.0264 mL | 0.1321 mL | 0.2643 mL | 0.5285 mL | 0.6606 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ambrisentan is a selective antagonist of ETA-receptor with Ki value of 1nM [1].
Ambrisentan is an orally active diphenyl propionic acid derivative. It is usually used to treat for pulmonary arterial hypertension. In the in vitro assay, ambrisentan shows selective affinity with ETA-receptor over ETB-receptor expressed in CHO cells. The Ki values of ETA- and ETB-receptor are 1nM and 195nM, respectively. This selectivity is much more higher for the recombinant human ET-receptors in intact cells. The Ki values of ETA- and ETB-receptor are 0.63nM and 48.7nM, respectively. As a selective antagonist of ETA-receptor, ambrisentan is preferential to non-selective receptor antagonism as it permitting maintenance of vasodilator and clearance functions specific to ETB- receptors on the endothelial cells. Moreover, ambrisentan also has possible use in the prevention of reperfusion injury and is appropriate to treat for cerebrovascular disorders [1, 2].
References:
[1] Vatter H, Seifert V. Ambrisentan, a Non-peptide Endothelin Receptor Antagonist. Cardiovascular drug reviews, 2006, 24(1): 63-76.
[2] Barst R J. A review of pulmonary arterial hypertension: role of ambrisentan. Vascular health and risk management, 2007, 3(1): 11.
- 2alpha,19alpha-Dihydroxy-3-oxo-urs-12-en-28-oic acid
Catalog No.:BCN7406
CAS No.:176983-21-4
- LY 320135
Catalog No.:BCC7346
CAS No.:176977-56-3
- CCT007093
Catalog No.:BCC5147
CAS No.:176957-55-4
- Metergoline
Catalog No.:BCC6709
CAS No.:17692-51-2
- Homoplantaginin
Catalog No.:BCN2488
CAS No.:17680-84-1
- (RS)-3,4-DCPG
Catalog No.:BCC7045
CAS No.:176796-64-8
- Ageratochromene dimer
Catalog No.:BCN8110
CAS No.:17678-76-1
- Neoruscogenin
Catalog No.:BCN8168
CAS No.:17676-33-4
- trans-Hinokiresinol
Catalog No.:BCN1130
CAS No.:17676-24-3
- Phorbol
Catalog No.:BCN3726
CAS No.:17673-25-5
- KN-92
Catalog No.:BCC1680
CAS No.:176708-42-2
- Delphinidin-3,5-O-diglucoside chloride
Catalog No.:BCN1522
CAS No.:17670-06-3
- R 568 hydrochloride
Catalog No.:BCC7781
CAS No.:177172-49-5
- 26-O-Acetylsootepin A
Catalog No.:BCN7699
CAS No.:1772588-99-4
- Aglain B
Catalog No.:BCN6636
CAS No.:177262-32-7
- DBeQ
Catalog No.:BCC3916
CAS No.:177355-84-9
- Aglain C
Catalog No.:BCN6604
CAS No.:177468-85-8
- 2-Amino-5-nitrobenzophenone
Catalog No.:BCC8537
CAS No.:1775-95-7
- Flavokawain B
Catalog No.:BCN3568
CAS No.:1775-97-9
- ZK 164015
Catalog No.:BCC7272
CAS No.:177583-70-9
- Glycerol 1-(26-hydroxyhexacosanoate)
Catalog No.:BCN1131
CAS No.:177602-14-1
- MNITMT
Catalog No.:BCC7382
CAS No.:177653-76-8
- NKP608
Catalog No.:BCC1802
CAS No.:177707-12-9
- Proxyfan oxalate
Catalog No.:BCC7378
CAS No.:177708-09-7
Combined approach using capillary electrophoresis, NMR and molecular modeling for ambrisentan related substances analysis: Investigation of intermolecular affinities, complexation and separation mechanism.[Pubmed:28131522]
J Pharm Biomed Anal. 2017 Sep 10;144:220-229.
A comprehensive investigation on the CE separation mechanisms and on the inclusion complexation with CyDs of the chiral drug S-Ambrisentan (S-AMB), its R-enantiomer and other impurities was performed by different techniques. A CE method was previously set up allowing the simultaneous determination of the enantiomeric purity and of impurities of S-AMB, based on the addition of SDS micelles and gamma-cyclodextrin (gammaCyD) to borate buffer. In this study, the electrophoretic behavior of the analytes in terms of selectivity and mobility with respect to the addition of different CyDs was first investigated, evidencing the presence of interactions for all the CyDs, but the unique ability of gammaCyD for obtaining the separation of all the compounds. By molecular modeling, aggregates between SDS micelles and analytes, and inclusion complexes between CyDs, SDS and/or analytes of different stoichiometries were simulated. The potential and the gain energy of complexes were calculated on the minimized conformations, showing the great tendency of gammaCyD of forming mixed complexes with one or two SDS molecules and with the analyte, even if with different affinities among the analytes. For 1:1:1 mixed complexes with different CyDs, the highest difference of potential energy between the enantiomers' complexes was observed for gammaCyD. Two-dimensional NOE spectroscopy experiments were performed for S-AMB and I1 and pointed out the interactions of the aromatic moiety of the analytes and of SDS aliphatic chain with gammaCyD protons, confirming the existence of gammaCyD mixed complexes. The high affinity of SDS for the gammaCyD cavity was suggested to justify the fundamental role of SDS in modulating and achieving the CE separation, due to its influence both on the stability and on the type of complexes between gammaCyD and the analytes.
Initial combination therapy with ambrisentan and tadalafil and mortality in patients with pulmonary arterial hypertension: a secondary analysis of the results from the randomised, controlled AMBITION study.[Pubmed:27745818]
Lancet Respir Med. 2016 Nov;4(11):894-901.
BACKGROUND: In treatment-naive patients with pulmonary arterial hypertension, initial combination therapy with Ambrisentan and tadalafil reduces the risk of clinical failure events compared with monotherapy. We did this secondary analysis to further investigate the effect of combination therapy on survival. METHODS: We analysed survival data from the modified intention-to-treat population of the Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension (AMBITION) trial. AMBITION was a multicentre, randomised, double-blind study, in which treatment-naive patients with pulmonary arterial hypertension were randomly assigned in a 2:1:1 ratio and received combination therapy with Ambrisentan and tadalafil, Ambrisentan and placebo, or tadalafil and placebo. We did a prespecified analysis of all mortality events from randomisation to the end of the study, including patients who discontinued their assigned treatment. In a post-hoc analysis, we analysed survival at 7 days after the termination of each individual patient's randomised treatment. We used Cox proportional hazard regression, Kaplan-Meier survival estimates, and the stratified log-rank test to compare the survival of patients receiving initial combination therapy or initial monotherapy. FINDINGS: The study population consisted of 605 patients with pulmonary arterial hypertension who were randomly assigned and received combination therapy (n=302) or monotherapy (n=303; 152 patients assigned to Ambrisentan monotherapy and 151 patients to tadalafil monotherapy). At the end of the study, 29 (10%) of 302 patients in the combination therapy group had died compared with 41 (14%) of 303 patients in the monotherapy group (hazard ratio 0.67, 95% CI 0.42-1.08; stratified log-rank p=0.10). At 7 days after the end of randomised treatment, fewer patients had died in the combination therapy group (3 [1%] of 302 patients) compared with the monotherapy group (13 [4%] of 303 patients; hazard ratio 0.21, 95% CI 0.06-0.73). INTERPRETATION: These data indicate that initial combination therapy might be associated with a survival advantage compared with initial monotherapy in patients with newly diagnosed pulmonary arterial hypertension. This hypothesis needs to be addressed in future studies. FUNDING: Gilead, GlaxoSmithKline.
Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial.[Pubmed:28039187]
Ann Rheum Dis. 2017 Jul;76(7):1219-1227.
BACKGROUND: Patients with connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH), in particular systemic sclerosis (SSc), had an attenuated response compared with idiopathic PAH in most trials. Thus, there is uncertainty regarding the benefit of PAH-targeted therapy in some forms of CTD-PAH. OBJECTIVE: To explore the safety and efficacy of initial combination therapy with Ambrisentan and tadalafil versus Ambrisentan or tadalafil monotherapy in patients with CTD-PAH and SSc-PAH enrolled in the AMBITION trial. METHODS: This was a post hoc analysis of patients with CTD-PAH and SSc-PAH from AMBITION, an event-driven, double-blind trial in patients with WHO functional class II/III PAH. Treatment-naive patients were randomised 2:1:1 to once-daily initial combination therapy with Ambrisentan plus tadalafil or monotherapy with Ambrisentan or tadalafil, respectively. The primary endpoint was time to the first clinical failure event (first occurrence of death, hospitalisation for worsening PAH, disease progression or unsatisfactory long-term clinical response). RESULTS: In the primary analysis set (N=500), 187 patients had CTD-PAH, of whom 118 had SSc-PAH. Initial combination therapy reduced the risk of clinical failure versus pooled monotherapy in each subgroup: CTD-PAH (HR 0.43 (95% CI 0.24 to 0.77)) and SSc-PAH (0.44 (0.22 to 0.89)). The most common AE was peripheral oedema, which was reported more frequently with initial combination therapy than monotherapy in the two PAH subgroups. The relative frequency of adverse events between those on combination therapy versus monotherapy was similar across subgroups. CONCLUSIONS: This post hoc subgroup analysis provides evidence that CTD-PAH and SSc-PAH patients benefit from initial Ambrisentan and tadalafil combination therapy. TRIAL REGISTRATION NUMBER: NCT01178073, post results.
Functional estimation of endothelin-1 receptor antagonism by bosentan, macitentan and ambrisentan in human pulmonary and radial arteries in vitro.[Pubmed:28300593]
Eur J Pharmacol. 2017 Jun 5;804:111-116.
BACKGROUND: Endothelin receptor antagonists are approved for pulmonary arterial hypertension. Development of selective ETA-receptor antagonists over mixed or dual receptor antagonists has depended on a range of receptor binding assays, second messenger assays and functional blood vessel assays. This study compared the 3 clinically-approved endothelin receptor antagonists in assays of human isolated pulmonary and radial arteries in vitro. METHODS: Human isolated pulmonary (i.d. 5.5mm) and human radial (i.d. 3.23mm) artery ring segments were mounted in organ baths for isometric force measurement. Single concentration-contraction curves to endothelin-1 were constructed in the absence or presence of bosentan (1-10microM), macitentan (0.03-0.3microM) or Ambrisentan (0.1-1microM). RESULTS: All 3 endothelin antagonists caused competitive rightward shifts in the endothelin-1 concentration-response curves in both arteries. The Clark plot and analysis gave the following pKB values: bosentan, pulmonary artery 6.28+/-0.13 and radial artery 6.04+/-0.10; macitentan, pulmonary artery 8.02+/-0.13 and radial artery 7.49+/-0.08; and Ambrisentan, pulmonary artery 7.38+/-0.13 and radial artery 6.96+/-0.10. CONCLUSIONS: Noting the maximum plasma levels attained from recommended oral doses of each antagonist in volunteers, the pKB findings here show that there would be significant antagonism of endothelin-1 contraction in the pulmonary and radial arteries at therapeutic plasma levels. This functional assay confirms in human tissue that much higher plasma concentrations of endothelin-1 receptor antagonists are required to be effective than those predicted from binding or other biochemical assays.


