KN-92CaMKII inhibitor CAS# 176708-42-2 |
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- A 438079 hydrochloride
Catalog No.:BCC1317
CAS No.:899431-18-6
- A 438079
Catalog No.:BCC1316
CAS No.:899507-36-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 176708-42-2 | SDF | Download SDF |
| PubChem ID | 5353702 | Appearance | Powder |
| Formula | C24H25ClN2O3S | M.Wt | 456.98 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >15.1mg/mL in DMSO | ||
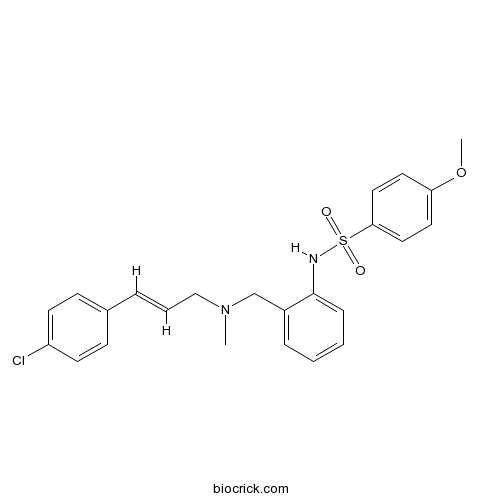
| Chemical Name | N-[2-[[[(E)-3-(4-chlorophenyl)prop-2-enyl]-methylamino]methyl]phenyl]-4-methoxybenzenesulfonamide | ||
| SMILES | CN(CC=CC1=CC=C(C=C1)Cl)CC2=CC=CC=C2NS(=O)(=O)C3=CC=C(C=C3)OC | ||
| Standard InChIKey | RUAOVVIUGUOYHA-AATRIKPKSA-N | ||
| Standard InChI | InChI=1S/C24H25ClN2O3S/c1-27(17-5-6-19-9-11-21(25)12-10-19)18-20-7-3-4-8-24(20)26-31(28,29)23-15-13-22(30-2)14-16-23/h3-16,26H,17-18H2,1-2H3/b6-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | KN-92 is an inactive derivative of KN-93. KN-93 is a selective inhibitor of Ca2+/calmodulin-dependent kinase II (CaMKII), competitively blocking CaM binding to the kinase (Ki = 370 nM).
IC50 value:
Target:
KN-92 is intended to be used as a control compound in studies designed to elucidate the antagonist activities of KN-93. KN-93 inhibits histamine-induced aminopyrine uptake in parietal cells (IC50 = 300 nM). KN-93 has been used to implicate roles for CaMKII in Ca2+-induced Ca2+ release in cardiac myocytes, constitutive phosphorylation of 5-lipoxygenase in 3T3 cells, and Ca2+-dependent activation of HIF-1α in colon cancer cell. References: | |||||

KN-92 Dilution Calculator

KN-92 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1883 mL | 10.9414 mL | 21.8828 mL | 43.7656 mL | 54.707 mL |
| 5 mM | 0.4377 mL | 2.1883 mL | 4.3766 mL | 8.7531 mL | 10.9414 mL |
| 10 mM | 0.2188 mL | 1.0941 mL | 2.1883 mL | 4.3766 mL | 5.4707 mL |
| 50 mM | 0.0438 mL | 0.2188 mL | 0.4377 mL | 0.8753 mL | 1.0941 mL |
| 100 mM | 0.0219 mL | 0.1094 mL | 0.2188 mL | 0.4377 mL | 0.5471 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KN-92 is an inactive derivative of KN-93. It is intended to be used as a control compound in studies designed to elucidate the antagonist activities of KN-93. KN-93 is a selective inhibitor of Ca2+/calmodulin-dependent kinase II (CaMKII), competitively blocking CaM binding to the kinase (Ki = 370 nM). KN-93 inhibits histamine-induced aminopyrine uptake in parietal cells (IC50 = 300 nM). KN-93 has been used to implicate roles for CaMKII in Ca2+-induced Ca2+ release in cardiac myocytes, constitutive phosphorylation of 5-lipoxygenase in 3T3 cells, and Ca2+-dependent activation of HIF-1α in colon cancer cell.
- Delphinidin-3,5-O-diglucoside chloride
Catalog No.:BCN1522
CAS No.:17670-06-3
- Ducheside A
Catalog No.:BCN1129
CAS No.:176665-78-4
- H-D-Leu-OBzl.TosOH
Catalog No.:BCC2976
CAS No.:17664-93-6
- Urocortin (human)
Catalog No.:BCC5788
CAS No.:176591-49-4
- AAL Toxin TE2
Catalog No.:BCN1742
CAS No.:176590-38-8
- AAL Toxin TE1
Catalog No.:BCN1737
CAS No.:176590-37-7
- AAL Toxin TD2
Catalog No.:BCN1740
CAS No.:176590-36-6
- AAL Toxin TD1
Catalog No.:BCN1735
CAS No.:176590-35-5
- AAL Toxin TC2
Catalog No.:BCN1741
CAS No.:176590-34-4
- AAL Toxin TC1
Catalog No.:BCN1736
CAS No.:176590-33-3
- Racanisodamine
Catalog No.:BCN8343
CAS No.:17659-49-3
- (+)-Taxifolin
Catalog No.:BCN5972
CAS No.:17654-26-1
- Phorbol
Catalog No.:BCN3726
CAS No.:17673-25-5
- trans-Hinokiresinol
Catalog No.:BCN1130
CAS No.:17676-24-3
- Neoruscogenin
Catalog No.:BCN8168
CAS No.:17676-33-4
- Ageratochromene dimer
Catalog No.:BCN8110
CAS No.:17678-76-1
- (RS)-3,4-DCPG
Catalog No.:BCC7045
CAS No.:176796-64-8
- Homoplantaginin
Catalog No.:BCN2488
CAS No.:17680-84-1
- Metergoline
Catalog No.:BCC6709
CAS No.:17692-51-2
- CCT007093
Catalog No.:BCC5147
CAS No.:176957-55-4
- LY 320135
Catalog No.:BCC7346
CAS No.:176977-56-3
- 2alpha,19alpha-Dihydroxy-3-oxo-urs-12-en-28-oic acid
Catalog No.:BCN7406
CAS No.:176983-21-4
- Ambrisentan
Catalog No.:BCC4887
CAS No.:177036-94-1
- R 568 hydrochloride
Catalog No.:BCC7781
CAS No.:177172-49-5
Constitutive regulation of the glutamate/aspartate transporter EAAT1 by Calcium-Calmodulin-Dependent Protein Kinase II.[Pubmed:27889915]
J Neurochem. 2017 Feb;140(3):421-434.
Glutamate clearance by astrocytes is an essential part of normal excitatory neurotransmission. Failure to adapt or maintain low levels of glutamate in the central nervous system is associated with multiple acute and chronic neurodegenerative diseases. The primary excitatory amino acid transporters in human astrocytes are EAAT1 and EAAT2 (GLAST and GLT-1, respectively, in rodents). While the inhibition of calcium/calmodulin-dependent kinase (CaMKII), a ubiquitously expressed serine/threonine protein kinase, results in diminished glutamate uptake in cultured primary rodent astrocytes (Ashpole et al. 2013), the molecular mechanism underlying this regulation is unknown. Here, we use a heterologous expression model to explore CaMKII regulation of EAAT1 and EAAT2. In transiently transfected HEK293T cells, pharmacological inhibition of CaMKII (using KN-93 or tat-CN21) reduces [(3) H]-glutamate uptake in EAAT1 without altering EAAT2-mediated glutamate uptake. While over-expressing the Thr287Asp mutant to enhance autonomous CaMKII activity had no effect on either EAAT1 or EAAT2-mediated glutamate uptake, over-expressing a dominant-negative version of CaMKII (Asp136Asn) diminished EAAT1 glutamate uptake. SPOTS peptide arrays and recombinant glutathione S-transferase-fusion proteins of the intracellular N- and C-termini of EAAT1 identified two potential phosphorylation sites at residues Thr26 and Thr37 in the N-terminus. Introducing an Ala (a non-phospho mimetic) at Thr37 diminished EAAT1-mediated glutamate uptake, suggesting that the phosphorylation state of this residue is important for constitutive EAAT1 function. Our study is the first to identify a glutamate transporter as a direct CaMKII substrate and suggests that CaMKII signaling is a critical driver of constitutive glutamate uptake by EAAT1.
Molecular Basis of Hypokalemia-Induced Ventricular Fibrillation.[Pubmed:26269574]
Circulation. 2015 Oct 20;132(16):1528-1537.
BACKGROUND: Hypokalemia is known to promote ventricular arrhythmias, especially in combination with class III antiarrhythmic drugs like dofetilide. Here, we evaluated the underlying molecular mechanisms. METHODS AND RESULTS: Arrhythmias were recorded in isolated rabbit and rat hearts or patch-clamped ventricular myocytes exposed to hypokalemia (1.0-3.5 mmol/L) in the absence or presence of dofetilide (1 mumol/L). Spontaneous early afterdepolarizations (EADs) and ventricular tachycardia/fibrillation occurred in 50% of hearts at 2.7 mmol/L [K] in the absence of dofetilide and 3.3 mmol/L [K] in its presence. Pretreatment with the Ca-calmodulin kinase II (CaMKII) inhibitor KN-93, but not its inactive analogue KN-92, abolished EADs and hypokalemia-induced ventricular tachycardia/fibrillation, as did the selective late Na current (INa) blocker GS-967. In intact hearts, moderate hypokalemia (2.7 mmol/L) significantly increased tissue CaMKII activity. Computer modeling revealed that EAD generation by hypokalemia (with or without dofetilide) required Na-K pump inhibition to induce intracellular Na and Ca overload with consequent CaMKII activation enhancing late INa and the L-type Ca current. K current suppression by hypokalemia and dofetilide alone in the absence of CaMKII activation were ineffective at causing EADs. CONCLUSIONS: We conclude that Na-K pump inhibition by even moderate hypokalemia plays a critical role in promoting EAD-mediated arrhythmias by inducing a positive feedback cycle activating CaMKII and enhancing late INa. Class III antiarrhythmic drugs like dofetilide sensitize the heart to this positive feedback loop.
[Regulation of S100B expression during long term potentiation].[Pubmed:25682687]
Ross Fiziol Zh Im I M Sechenova. 2014 Aug;100(8):953-63.
In this study, contributions of intracellular regulatory cascades in the induction of S100B expression in rat hippocampal CA1 area during long term posttetanic potentiation (LTP) were estimated. The activation of transcription factor p53 (positive regulator of S100B transcription) by nutlin-3 increased the basal content of S100B mRNA up to 151% of the control level, which was significantly lower than its content in tetanized slices (280%). Therefore, p53 seems to be not unique transcription factor upregulating S100B expression during LTP. The inhibitor of Ca2+/calmodulin-dependent kinases (CaMKs) KN-93 fully blocked the increase of S100B mRNA after tetanization, while KN-92 (inactive analogue of KN-93) was ineffective. The inhibitor of CaMKII and receptor tyrosine kinases K-252a essentially suppressed S100B expression during LTP, the inhibition of MAPK p38 or RSK2 moderately decreased, and the inhibition of MEK1 did not influence S100B mRNA content. Thus, CaMKs play a key role in the induction of S100B expression during LTP.
Regulation of voltage-gated Ca(2+) currents by Ca(2+)/calmodulin-dependent protein kinase II in resting sensory neurons.[Pubmed:25064143]
Mol Cell Neurosci. 2014 Sep;62:10-8.
Calcium/calmodulin-dependent protein kinase II (CaMKII) is recognized as a key element in encoding depolarization activity of excitable cells into facilitated voltage-gated Ca(2+) channel (VGCC) function. Less is known about the participation of CaMKII in regulating VGCCs in resting cells. We examined constitutive CaMKII control of Ca(2+) currents in peripheral sensory neurons acutely isolated from dorsal root ganglia (DRGs) of adult rats. The small molecule CaMKII inhibitor KN-93 (1.0muM) reduced depolarization-induced ICa by 16-30% in excess of the effects produced by the inactive homolog KN-92. The specificity of CaMKII inhibition on VGCC function was shown by the efficacy of the selective CaMKII blocking peptide autocamtide-2-related inhibitory peptide in a membrane-permeable myristoylated form, which also reduced VGCC current in resting neurons. Loss of VGCC currents is primarily due to reduced N-type current, as application of mAIP selectively reduced N-type current by approximately 30%, and prior N-type current inhibition eliminated the effect of mAIP on VGCCs, while prior block of L-type channels did not reduce the effect of mAIP on total ICa. T-type currents were not affected by mAIP in resting DRG neurons. Transduction of sensory neurons in vivo by DRG injection of an adeno-associated virus expressing AIP also resulted in a loss of N-type currents. Together, these findings reveal a novel molecular adaptation whereby sensory neurons retain CaMKII support of VGCCs despite remaining quiescent.
Apoptosis induced by NAD depletion is inhibited by KN-93 in a CaMKII-independent manner.[Pubmed:26024774]
Exp Cell Res. 2015 Jul 1;335(1):62-7.
Nicotinamide phosphoribosyltransferase (NAMPT) is a key enzyme that catalyzes the synthesis of nicotinamide mononucleotide from nicotinamide (Nam) in the salvage pathway of mammalian NAD biosynthesis. Several potent NAMPT inhibitors have been identified and used to investigate the role of intracellular NAD and to develop therapeutics. NAD depletion induced by NAMPT inhibitors depolarizes mitochondrial membrane potential and causes apoptosis in a range of cell types. However, the mechanisms behind this depolarization have not been precisely elucidated. We observed that apoptosis of THP-1 cells in response to NAMPT inhibitors was reduced by the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) inhibitor KN-93 via an unknown mechanism. The inactive analog of KN-93, KN-92, exhibited the same activity, but the CaMKII-inhibiting cell-permeable autocamtide-2-related inhibitory peptide II did not, indicating that the inhibition of THP-1 cell apoptosis was not dependent on CaMKII. In evaluating the mechanism of action, we confirmed that KN-93 did not inhibit decreases in NAD levels but did inhibit decreases in mitochondrial membrane potential, indicating that KN-93 exerts inhibition upstream of the mitochondrial pathway of apoptosis. Further, qPCR analysis of the Bcl-2 family of proteins showed that Bim is efficiently expressed following NAMPT inhibition and that KN-92 did not inhibit this expression. The L-type Ca(2+) channel blockers verapamil and nimodipine partially inhibited apoptosis, indicating that part of this effect is dependent on Ca(2+) channel inhibition, as both KN-93 and KN-92 are reported to inhibit L-type Ca(2+) channels. On the other hand, KN-93 and KN-92 did not markedly inhibit apoptosis induced by anti-cancer agents such as etoposide, actinomycin D, ABT-737, or TW-37, indicating that the mechanism of inhibition is specific to apoptosis induced by NAD depletion. These results demonstrate that NAD depletion induces a specific type of apoptosis that is effectively inhibited by the KN-93 series of compounds.


