AAL Toxin TC2CAS# 176590-34-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 176590-34-4 | SDF | Download SDF |
| PubChem ID | 102004454 | Appearance | Powder |
| Formula | C25H47NO8 | M.Wt | 489.65 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
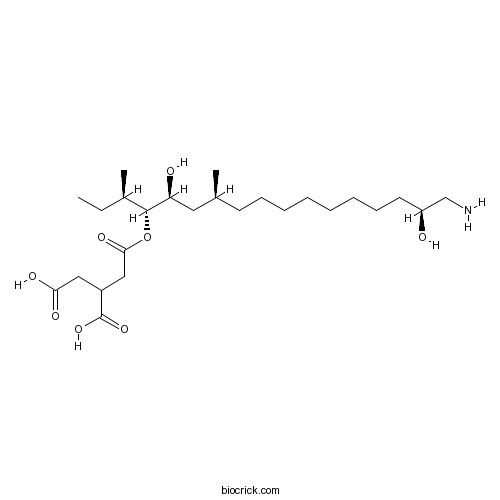
| Chemical Name | 2-[2-[(3R,4R,5S,7S,16S)-17-amino-5,16-dihydroxy-3,7-dimethylheptadecan-4-yl]oxy-2-oxoethyl]butanedioic acid | ||
| SMILES | CCC(C)C(C(CC(C)CCCCCCCCC(CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O | ||
| Standard InChIKey | JAUSVHGUDAUTEZ-YAUBIBOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. AAL-toxin has a wide range of phytotoxicity, it has potential as a natural herbicide because several important weeds including jimsonweed, black nightshade, prickly sida and hemp sesbania are quite sensitive, while some crops such as cotton and maize are not affected. |
| Targets | Antifection |

AAL Toxin TC2 Dilution Calculator

AAL Toxin TC2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0423 mL | 10.2114 mL | 20.4228 mL | 40.8455 mL | 51.0569 mL |
| 5 mM | 0.4085 mL | 2.0423 mL | 4.0846 mL | 8.1691 mL | 10.2114 mL |
| 10 mM | 0.2042 mL | 1.0211 mL | 2.0423 mL | 4.0846 mL | 5.1057 mL |
| 50 mM | 0.0408 mL | 0.2042 mL | 0.4085 mL | 0.8169 mL | 1.0211 mL |
| 100 mM | 0.0204 mL | 0.1021 mL | 0.2042 mL | 0.4085 mL | 0.5106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AAL Toxin TC1
Catalog No.:BCN1736
CAS No.:176590-33-3
- Racanisodamine
Catalog No.:BCN8343
CAS No.:17659-49-3
- (+)-Taxifolin
Catalog No.:BCN5972
CAS No.:17654-26-1
- Scutebarbatine A
Catalog No.:BCN1128
CAS No.:176520-13-1
- 8-Hydroxyodoroside A
Catalog No.:BCN1127
CAS No.:176519-75-8
- Nicotiflorin
Catalog No.:BCN1126
CAS No.:17650-84-9
- 16 alpha-Hydroxytrametenolic acid
Catalog No.:BCN2917
CAS No.:176390-68-4
- 16alpha-Hydroxydehydrotrametenolic acid
Catalog No.:BCN1523
CAS No.:176390-66-2
- Fmoc-D-Thr-ol
Catalog No.:BCC2575
CAS No.:176380-53-3
- Dihydropinosylvin methyl ether
Catalog No.:BCN1125
CAS No.:17635-59-5
- D-Raffinose Pentahydrate
Catalog No.:BCN2567
CAS No.:17629-30-0
- Nervogenic acid
Catalog No.:BCN1124
CAS No.:17622-86-5
- AAL Toxin TD1
Catalog No.:BCN1735
CAS No.:176590-35-5
- AAL Toxin TD2
Catalog No.:BCN1740
CAS No.:176590-36-6
- AAL Toxin TE1
Catalog No.:BCN1737
CAS No.:176590-37-7
- AAL Toxin TE2
Catalog No.:BCN1742
CAS No.:176590-38-8
- Urocortin (human)
Catalog No.:BCC5788
CAS No.:176591-49-4
- H-D-Leu-OBzl.TosOH
Catalog No.:BCC2976
CAS No.:17664-93-6
- Ducheside A
Catalog No.:BCN1129
CAS No.:176665-78-4
- Delphinidin-3,5-O-diglucoside chloride
Catalog No.:BCN1522
CAS No.:17670-06-3
- KN-92
Catalog No.:BCC1680
CAS No.:176708-42-2
- Phorbol
Catalog No.:BCN3726
CAS No.:17673-25-5
- trans-Hinokiresinol
Catalog No.:BCN1130
CAS No.:17676-24-3
- Neoruscogenin
Catalog No.:BCN8168
CAS No.:17676-33-4
AAL toxins, fumonisins (biology and chemistry) and host-specificity concepts.[Pubmed:1513374]
Mycopathologia. 1992 Feb;117(1-2):47-56.
The AAL toxins(include AAL Toxin TC2) and the fumonisins (FB1 and FB2) are structurally related and produced respectively by Alternaria alternata f.sp. lycopersici and Fusarium moniliforme. AAL toxin(include AAL Toxin TC2) is characterized as a host-specific toxin, toxic to tomato, whereas fumonisin B1 causes equine leukoencephalomalacia. FB1 and FB2 were biologically active in susceptible tomato tissue (Earlypak-7) and animal tissue culture (rat hepatoma H4TG and dog kidney MDCK). Conversely, AAL toxin was also active in the rat and dog tissue culture cells. Both fungi produce toxin/s in culture that causes death in rats; these toxins are other than AAL and fumonisin. The peracetylated derivatives of AAL and FB1 are biologically inactive in both the tomato bioassay and the animal tissue culture systems. Acetylation of the amine renders AAL inactive. The hydrolysis product of AAL (phentolamine) is toxic to the susceptible tomato line whereas the phentolamine of fumonisin is not. AAL and FB1 can be analyzed by Continuous Flow Fast Atom Bombardment (CFFAB) and Ionspray Mass Spectrometry (ISM), both sensitive to the picomole range. The N-acetyl of the TFA hydrolysis product of AAL and FB1 is determined by comparing the fragment ions at m/z 86 and 140 for FB1 and 72 and 126 for AAL.
Alternaria toxin-induced resistance in rose plants against rose aphid (Macrosiphum rosivorum): effect of tenuazonic acid.[Pubmed:25845360]
J Zhejiang Univ Sci B. 2015 Apr;16(4):264-74.
Many different types of toxins are produced by the fungus, Alternaria alternata (Fr.) Keissler. Little is known, however, regarding the influence of these toxins on insects. In this study, we investigated the toxin-induced inhibitory effects of the toxin(include AAL Toxin TC2) produced by A. alternata on the rose aphid, Macrosiphum rosivorum, when the toxin was applied to leaves of the rose, Rosa chinensis. The results demonstrated that the purified crude toxin was non-harmful to rose plants and rose aphids, but had an intensive inhibitory effect on the multiplication of aphids. The inhibitory index against rose aphids reached 87.99% when rose plants were sprayed with the toxin solution at a low concentration. Further results from bioassays with aphids and high performance liquid chromatography (HPLC) analyses demonstrated that tenuazonic acid (TeA) was one of the most important resistance-related active components in the crude toxin. The content of TeA was 0.1199% in the crude toxin under the HPLC method. Similar to the crude toxin, the inhibitory index of pure TeA reached 83.60% 15 d after the rose plants were sprayed with pure TeA solution at the lower concentration of 0.060 μg/ml, while the contents of residual TeA on the surface and in the inner portion of the rose plants were only 0.04 and 0.00 ng/g fresh weight of TeA-treated rose twigs, respectively, 7 d after the treatment. Our results show that TeA, an active component in the A. alternata toxin, can induce the indirect plant-mediated responses in rose plants to intensively enhance the plant's resistances against rose aphids, and the results are very helpful to understand the plant-mediated interaction between fungi and insects on their shared host plants.


