Nervogenic acidCAS# 17622-86-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17622-86-5 | SDF | Download SDF |
| PubChem ID | 5320129 | Appearance | Powder |
| Formula | C17H22O3 | M.Wt | 274.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
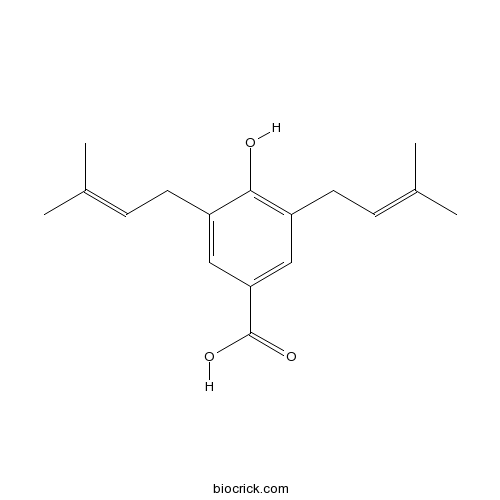
| Chemical Name | 4-hydroxy-3,5-bis(3-methylbut-2-enyl)benzoic acid | ||
| SMILES | CC(=CCC1=CC(=CC(=C1O)CC=C(C)C)C(=O)O)C | ||
| Standard InChIKey | LSVOBJIOONAGLU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H22O3/c1-11(2)5-7-13-9-15(17(19)20)10-14(16(13)18)8-6-12(3)4/h5-6,9-10,18H,7-8H2,1-4H3,(H,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Nervogenic acid shows good pro-coagulant activity in vitro. 2. Nervogenic acid displays significant antibacterial activities. 3. Nervogenic acid has antioxidative activity, it exhibits higher activity than that of t-butyl-4- hydroxyanisole (BHA) using the ferric thiocyanate method. |
| Targets | Antifection |

Nervogenic acid Dilution Calculator

Nervogenic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6443 mL | 18.2216 mL | 36.4431 mL | 72.8863 mL | 91.1079 mL |
| 5 mM | 0.7289 mL | 3.6443 mL | 7.2886 mL | 14.5773 mL | 18.2216 mL |
| 10 mM | 0.3644 mL | 1.8222 mL | 3.6443 mL | 7.2886 mL | 9.1108 mL |
| 50 mM | 0.0729 mL | 0.3644 mL | 0.7289 mL | 1.4577 mL | 1.8222 mL |
| 100 mM | 0.0364 mL | 0.1822 mL | 0.3644 mL | 0.7289 mL | 0.9111 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- LY 354740
Catalog No.:BCC7614
CAS No.:176199-48-7
- Depressine
Catalog No.:BCN7851
CAS No.:176182-06-2
- Maribavir
Catalog No.:BCC5259
CAS No.:176161-24-3
- H-Val-OEt.HCl
Catalog No.:BCC3141
CAS No.:17609-47-1
- Zerumin A
Catalog No.:BCN3684
CAS No.:176050-48-9
- Fucosterol
Catalog No.:BCN6427
CAS No.:17605-67-3
- (±)-Sigmoidin A
Catalog No.:BCN3372
CAS No.:176046-04-1
- Valganciclovir HCl
Catalog No.:BCC4745
CAS No.:175865-59-5
- Wedelialactone A
Catalog No.:BCN6733
CAS No.:175862-40-5
- H-Dab.HBr
Catalog No.:BCC3184
CAS No.:1758-80-1
- PPAHV
Catalog No.:BCC7077
CAS No.:175796-50-6
- Lanatoside C
Catalog No.:BCN6457
CAS No.:17575-22-3
- D-Raffinose Pentahydrate
Catalog No.:BCN2567
CAS No.:17629-30-0
- Dihydropinosylvin methyl ether
Catalog No.:BCN1125
CAS No.:17635-59-5
- Fmoc-D-Thr-ol
Catalog No.:BCC2575
CAS No.:176380-53-3
- 16alpha-Hydroxydehydrotrametenolic acid
Catalog No.:BCN1523
CAS No.:176390-66-2
- 16 alpha-Hydroxytrametenolic acid
Catalog No.:BCN2917
CAS No.:176390-68-4
- Nicotiflorin
Catalog No.:BCN1126
CAS No.:17650-84-9
- 8-Hydroxyodoroside A
Catalog No.:BCN1127
CAS No.:176519-75-8
- Scutebarbatine A
Catalog No.:BCN1128
CAS No.:176520-13-1
- (+)-Taxifolin
Catalog No.:BCN5972
CAS No.:17654-26-1
- Racanisodamine
Catalog No.:BCN8343
CAS No.:17659-49-3
- AAL Toxin TC1
Catalog No.:BCN1736
CAS No.:176590-33-3
- AAL Toxin TC2
Catalog No.:BCN1741
CAS No.:176590-34-4
New nervogenic acid derivatives from Liparis nervosa.[Pubmed:23322560]
Planta Med. 2013 Mar;79(3-4):281-7.
Ten new Nervogenic acid derivatives (1-4, 6-11) and one known compound (5) have been isolated from Liparis nervosa. Their structures were determined using extensive spectroscopic analysis, including 1D and 2D NMR experiments. Compounds 3, 4, 9, 10, and 11 were evaluated for their cytotoxicity against A549, H460, Hela, MCF-7, Caco2, and HepG2 human cancer cell lines.
Five new prenylated p-hydroxybenzoic acid derivatives with antimicrobial and molluscicidal activity from Piper aduncum leaves.[Pubmed:8302955]
Planta Med. 1993 Dec;59(6):546-51.
Five new prenylated benzoic acid derivatives, methyl 3-(3,7-dimethyl-2,6-octadienyl)-4-methoxybenzoate (1), 1-(1-methylethyl)-4-methyl-3-cyclohexenyl 3,5-bis(3-methyl-2-butenyl)-4-hydroxybenzoate (2), 1-(1-methylethyl)-4-methyl-3-cyclohexenyl 3,5-bis(3-methyl-2-butenyl)-4-methoxybenzoate (3), methyl 3,5-bis(3-methyl-2-butenyl)-4-methoxybenzoate (4), and 4-hydroxy-3-(3-methyl-2-butenyl)-5-(3-methyl-2-butenyl)-benzoic acid (5) were isolated from the dried leaves of Piper aduncum L. (Piperaceae). Together with the new metabolites, four known prenylated benzoic acid derivatives, 3,5-bis(3-methyl-2-butenyl)-4-methoxybenzoic acid (6), 4-hydroxy-3,5-bis(3-methyl-2-butenyl)-benzoic acid (Nervogenic acid, 7), methyl 4-hydroxy-3,5-bis(3-methyl-2-butenyl)-benzoate (8), and methyl 4-hydroxy-3-(3-methyl-2-butenyl)-benzoate (9) as well as, dillapiol (10), myristicin, and the three sesquiterpenes humulene, caryophyllene epoxide, and humulene epoxide were isolated. Compounds 7, 8, and 9 are reported as natural products for the first time. The structures of the isolates were elucidated by spectroscopic methods, mainly 1D-and 2D-NMR spectroscopy. Isolates 4-7, 9, and 10 were molluscicidal while 2, 5-7, and 9 displayed significant antibacterial activities.
A new nervogenic acid glycoside with pro-coagulant activity from Liparis nervosa.[Pubmed:24079181]
Nat Prod Commun. 2013 Aug;8(8):1115-6.
In an effort to identify hemostatic components from Liparis nervosa (Thunb.) Lindl. using a bioactivity-guided fractionation approach, the n-BuOH extract was found to promote ADP-induced platelet aggregation and two compounds were isolated from the active extract. Compound 1 was a new Nervogenic acid glycoside and the structure was elucidated as 3,5-bis(3-methyl-but-2-enyl)-4-O-[beta-D-xylopyranosyl-(1 -->2)-beta-D-glucopyranosyl]-benzoic acid by extensive spectroscopic measurements. Adenosine (2) was isolated from this plant for the first time. Compound 1 also showed good pro-coagulant activity in vitro.
Glycosylated nervogenic acid derivatives from Liparis condylobulbon (Reichb.f.) leaves.[Pubmed:19664759]
Carbohydr Res. 2009 Sep 8;344(13):1770-4.
Three new Nervogenic acid glycosides, 1-O-alpha-L-rhamnopyranosyl 3,5-bis(3-methyl-but-2-enyl)-4-O-[alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyr anosyl]-benzoate, 3,5-bis(3-methyl-but-2-enyl)-4-O-[alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyr anosyl]-benzoic acid, and bis{3,5-bis(3-methyl-but-2-enyl)-4-O-[alpha-L-rhamnopyranosyl-(1-->2)-beta-D-gluc opyranosyl]-benzoyl} 1,2-O-beta-d-glucopyranose, which we named condobulbosides A-C, were isolated from a methanol extract of the leaves of Liparis condylobulbon together with an apigenin C-glycoside, schaftoside. Their structures were established on the basis of spectral techniques, namely, UV, IR, HR-MS spectroscopy, both 1D and 2D NMR experiments, and chemical reactions.


