NicotiflorinCAS# 17650-84-9 |
- Biorobin
Catalog No.:BCN4691
CAS No.:17297-56-2
Quality Control & MSDS
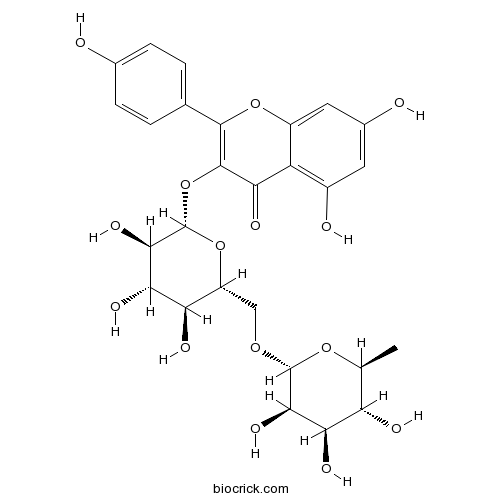
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17650-84-9 | SDF | Download SDF |
| PubChem ID | 5318767 | Appearance | Yellow powder |
| Formula | C27H30O15 | M.Wt | 594.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Nicotifiorin; Nicotiflorin; Nicotifloroside; 3,4',5,7-Tetrahydroxyflavone 3-rutinoside | ||
| Solubility | DMSO : 100 mg/mL (168.20 mM; Need ultrasonic) | ||
| Chemical Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=C(OC4=CC(=CC(=C4C3=O)O)O)C5=CC=C(C=C5)O)O)O)O)O)O)O | ||
| Standard InChIKey | RTATXGUCZHCSNG-QHWHWDPRSA-N | ||
| Standard InChI | InChI=1S/C27H30O15/c1-9-17(31)20(34)22(36)26(39-9)38-8-15-18(32)21(35)23(37)27(41-15)42-25-19(33)16-13(30)6-12(29)7-14(16)40-24(25)10-2-4-11(28)5-3-10/h2-7,9,15,17-18,20-23,26-32,34-37H,8H2,1H3/t9-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nicotiflorin shows potent antiglycation activity and neuroprotection effects, it has protective effects on cerebral ischemic damage, reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. |
| Targets | NOS | ATPase | Calcium Channel | Potassium Channel |
| In vitro | Nicotiflorin, rutin and chlorogenic acid: phenylpropanoids involved differently in quantitative resistance of potato tubers to biotrophic and necrotrophic pathogens.[Pubmed: 22677447]Plant Physiol Biochem. 2012 Aug;57:23-31.Physiological and molecular mechanisms underlying quantitative resistance of plants to pathogens are still poorly understood, but could depend upon differences in the intensity or timing of general defense responses.
|
| In vivo | Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats.[Pubmed: 17448528]Pharmacol Biochem Behav. 2007 Apr;86(4):741-8.The present study aimed to determine whether Nicotiflorin, a natural flavonoid extracted from coronal of Carthamus tinctorius, has a protective effect on cerebral multi-infarct dementia in rats.
|
| Cell Research | Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells.[Pubmed: 16806761]J Ethnopharmacol. 2006 Aug 11;107(1):143-50.Nicotiflorin is a flavonoid glycoside extracted from a traditional Chinese medicine Flos Carthami.
|
| Structure Identification | Med Chem. 2012 May;8(3):415-20.Synthesis and antiglycation activity of kaempferol-3-O-rutinoside (nicotiflorin).[Pubmed: 22530897]
|

Nicotiflorin Dilution Calculator

Nicotiflorin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6821 mL | 8.4104 mL | 16.8209 mL | 33.6417 mL | 42.0521 mL |
| 5 mM | 0.3364 mL | 1.6821 mL | 3.3642 mL | 6.7283 mL | 8.4104 mL |
| 10 mM | 0.1682 mL | 0.841 mL | 1.6821 mL | 3.3642 mL | 4.2052 mL |
| 50 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6728 mL | 0.841 mL |
| 100 mM | 0.0168 mL | 0.0841 mL | 0.1682 mL | 0.3364 mL | 0.4205 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 16 alpha-Hydroxytrametenolic acid
Catalog No.:BCN2917
CAS No.:176390-68-4
- 16alpha-Hydroxydehydrotrametenolic acid
Catalog No.:BCN1523
CAS No.:176390-66-2
- Fmoc-D-Thr-ol
Catalog No.:BCC2575
CAS No.:176380-53-3
- Dihydropinosylvin methyl ether
Catalog No.:BCN1125
CAS No.:17635-59-5
- D-Raffinose Pentahydrate
Catalog No.:BCN2567
CAS No.:17629-30-0
- Nervogenic acid
Catalog No.:BCN1124
CAS No.:17622-86-5
- LY 354740
Catalog No.:BCC7614
CAS No.:176199-48-7
- Depressine
Catalog No.:BCN7851
CAS No.:176182-06-2
- Maribavir
Catalog No.:BCC5259
CAS No.:176161-24-3
- H-Val-OEt.HCl
Catalog No.:BCC3141
CAS No.:17609-47-1
- Zerumin A
Catalog No.:BCN3684
CAS No.:176050-48-9
- Fucosterol
Catalog No.:BCN6427
CAS No.:17605-67-3
- 8-Hydroxyodoroside A
Catalog No.:BCN1127
CAS No.:176519-75-8
- Scutebarbatine A
Catalog No.:BCN1128
CAS No.:176520-13-1
- (+)-Taxifolin
Catalog No.:BCN5972
CAS No.:17654-26-1
- Racanisodamine
Catalog No.:BCN8343
CAS No.:17659-49-3
- AAL Toxin TC1
Catalog No.:BCN1736
CAS No.:176590-33-3
- AAL Toxin TC2
Catalog No.:BCN1741
CAS No.:176590-34-4
- AAL Toxin TD1
Catalog No.:BCN1735
CAS No.:176590-35-5
- AAL Toxin TD2
Catalog No.:BCN1740
CAS No.:176590-36-6
- AAL Toxin TE1
Catalog No.:BCN1737
CAS No.:176590-37-7
- AAL Toxin TE2
Catalog No.:BCN1742
CAS No.:176590-38-8
- Urocortin (human)
Catalog No.:BCC5788
CAS No.:176591-49-4
- H-D-Leu-OBzl.TosOH
Catalog No.:BCC2976
CAS No.:17664-93-6
Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats.[Pubmed:17448528]
Pharmacol Biochem Behav. 2007 Apr;86(4):741-8.
The present study aimed to determine whether Nicotiflorin, a natural flavonoid extracted from coronal of Carthamus tinctorius, has a protective effect on cerebral multi-infarct dementia in rats. The multi-infarct dementia model rats were prepared by injecting man-made micro-thrombi into the right hemisphere. The administration groups were treated once daily with 30, 60 and 120 mg/kg Nicotiflorin (i.g.) from 5 days before ischemia operation to 3 days after the operation for biochemical examination, 10 days for Morris water maze study and morphological observations and 20 days for eight-arm radial maze task. 2,3,5-triphenyltetrazolium chloride (TTC) staining showed that infarct volume of each Nicotiflorin administration group was much smaller than that of vehicle-treated multi-infarct dementia group, and hematoxylin and eosin (HE) staining showed that histopathological abnormalities of each Nicotiflorin group were also much lighter than that of vehicle-treated multi-infarct dementia group. Each Nicotiflorin group showed much better spatial memory performance in Morris water maze tests and eight-arm radial maze task compared with the vehicle-treated multi-infarct dementia group, significantly attenuated the elevation of lactic acid and malondialdehyde (MDA) contents and the decrease in lactate dehydrogenase (LDH), Na(+)K(+)ATPase, Ca(2+)Mg(2+)ATPase and superoxide dismutase (SOD) activity in the brain tissue which was composed of striatum, cortex and hippocampus of the ischemia hemisphere at day 3 after ischemia operation. These results suggest that Nicotiflorin has protective effects on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats.
Isolation and characterization of nicotiflorin obtained by enzymatic hydrolysis of two precursors in tea seed extract.[Pubmed:20225859]
J Agric Food Chem. 2010 Apr 28;58(8):4808-13.
Two flavonol triglycosides, camelliaside A (CamA) and camelliaside B (CamB), of tea seed extract (TSE) were subjected to enzymatic hydrolysis. Among five kinds of glycosidases investigated, beta-galactosidase (Gal) induced selective hydrolysis of CamA. On the other hand, pectinase (Pec) and cellulase (Cel) induced hydrolysis of CamB. For Gal and Pec, only kaempferol diglycoside (Nicotiflorin, NF) was produced; on the other hand, significant amounts of kaempferol monoglycoside (astragalin, AS) and kaempferol (KR) were also detected for Cel. The combination of the use of Gal and Pec in the enzymatic hydrolysis of TSE afforded NF with high specificity. Crude NF with 22% purity was recovered from the enzymatic reaction mixture by extraction with organic solvent, and pure NF with >95% purity was obtained by crystallized in water. The chemical structure of NF was confirmed by (1)H and (13)C NMR analyses.
Nicotiflorin, rutin and chlorogenic acid: phenylpropanoids involved differently in quantitative resistance of potato tubers to biotrophic and necrotrophic pathogens.[Pubmed:22677447]
Plant Physiol Biochem. 2012 Aug;57:23-31.
Physiological and molecular mechanisms underlying quantitative resistance of plants to pathogens are still poorly understood, but could depend upon differences in the intensity or timing of general defense responses. This may be the case for the biosynthesis of phenolics which are known to increase after elicitation by pathogens. We thus tested the hypothesis that differences in quantitative resistance were related to differential induction of phenolics by pathogen-derived elicitors. Five potato cultivars (Solanum tuberosum, L.) spanning a range of quantitative resistance were treated with a concentrated culture filtrate (CCF) of Phytophthora infestans or purified lipopolysaccharides (LPS) from Pectobacterium atrosepticum. The kinetic of phenolics accumulation was followed and a set of typical phenolics was identified: chlorogenic acid, phenolamides and flavonols including rutin (quercetin-3-O-rutinoside) and Nicotiflorin (kaempferol-3-O-rutinoside). Our results showed that CCF but not LPS induced differential accumulation of major phenolics among cultivars. Total phenolics were related with resistance to P. atrosepticum but not to P. infestans. However, Nicotiflorin was inversely related with resistance to both pathogens. Rutin, but not Nicotiflorin, inhibited pathogen growth in vitro at physiological concentrations. These data therefore suggest that (i) several phenolics are candidate markers for quantitative resistance in potato, (ii) some of these are pathogen specific although they are produced by a general defense pathway, (iii) resistance marker molecules do not necessarily have antimicrobial activity, and (iv) the final content of these target molecules-either constitutive or induced-is a better predictor of resistance than their inducibility by pathogen elicitors.
Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells.[Pubmed:16806761]
J Ethnopharmacol. 2006 Aug 11;107(1):143-50.
Nicotiflorin is a flavonoid glycoside extracted from a traditional Chinese medicine Flos Carthami. In the current study, we investigated the neuroprotective effect of Nicotiflorin on a transient focal cerebral ischemia-reperfusion model in rats. Nicotiflorin (2.5-10 mg/kg) administered after onset of ischemia markedly reduced brain infarct volume by 24.5-63.2% and neurological deficits. Also the effect of Nicotiflorin on endothelial nitric oxide synthase (eNOS) activity, mRNA and protein expression after hypoxia-reoxygenation (H-R) treatment was investigated in an in vitro model mimic cerebrum ischemia-reperfusion in vivo. After total 4 h hypoxia and 12 h reoxygenation, eNOS activity, mRNA and protein levels in the primarily cultured rat cerebral blood vessel endothelial cells treated with Nicotiflorin (25-100 microg/ml) 2 h after onset of hypoxia were significantly higher than eNOS activity, mRNA and protein levels in the pure H-R cells and also higher than eNOS activity, mRNA and protein levels in cells cultured under normoxic conditions. The results demonstrated that Nicotiflorin had a protective effect against cerebral ischemic damage. The results also gave an important elucidation for the mechanism underlying the protective effect at the cellular level.


