A 438079 hydrochlorideP2X7 receptor antagonist,competitive and selective CAS# 899431-18-6 |
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- KN-92 hydrochloride
Catalog No.:BCC1681
CAS No.:1431698-47-3
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- A 438079
Catalog No.:BCC1316
CAS No.:899507-36-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 899431-18-6 | SDF | Download SDF |
| PubChem ID | 11688742 | Appearance | Powder |
| Formula | C13H10Cl3N5 | M.Wt | 342.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 350 mg/mL (1021.57 mM) DMSO : ≥ 100 mg/mL (291.88 mM) *"≥" means soluble, but saturation unknown. | ||
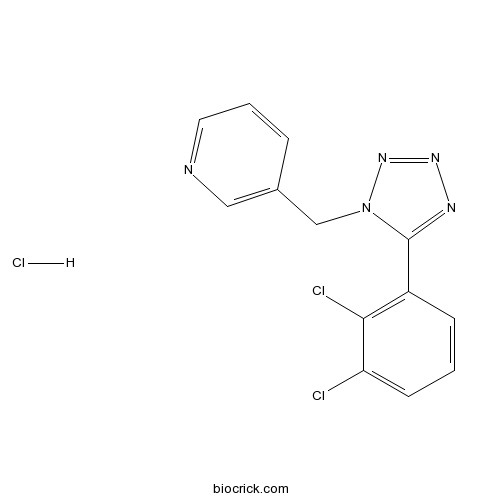
| Chemical Name | 3-[[5-(2,3-dichlorophenyl)tetrazol-1-yl]methyl]pyridine;hydrochloride | ||
| SMILES | C1=CC(=C(C(=C1)Cl)Cl)C2=NN=NN2CC3=CN=CC=C3.Cl | ||
| Standard InChIKey | MBTJFFMIPPMRGR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H9Cl2N5.ClH/c14-11-5-1-4-10(12(11)15)13-17-18-19-20(13)8-9-3-2-6-16-7-9;/h1-7H,8H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive P2X7 receptor antagonist (pIC50 = 6.9 for the inhibition of Ca2+ influx in the human recombinant P2X7 cell line). Devoid of activity at other P2 receptors (IC50 >> 10 μM). Possesses antinociceptive activity in models of neuropathic pain in vivo. |

A 438079 hydrochloride Dilution Calculator

A 438079 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9188 mL | 14.5939 mL | 29.1877 mL | 58.3754 mL | 72.9693 mL |
| 5 mM | 0.5838 mL | 2.9188 mL | 5.8375 mL | 11.6751 mL | 14.5939 mL |
| 10 mM | 0.2919 mL | 1.4594 mL | 2.9188 mL | 5.8375 mL | 7.2969 mL |
| 50 mM | 0.0584 mL | 0.2919 mL | 0.5838 mL | 1.1675 mL | 1.4594 mL |
| 100 mM | 0.0292 mL | 0.1459 mL | 0.2919 mL | 0.5838 mL | 0.7297 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A 438079 hydrochloride is a selective antagonist of P2X7 receptor with IC50 values of 100 and 300 nM in rat and human, respectively [1].
A 438079 is a derivative of the origin compound tetrazole 5 selected out as a novel P2X7 receptor antagonist. In human 1321N1 astrocytoma cells with expressing recombinant human P2X7, A 438079 treatment suppressed the Ca 2+ flux with pIC50 value of 6.9. In the pore formation assay, A 438079 blocked the pore formation of human THP-1 cells and prevented the cells from uptaking the diiodide dye YO-PRO. Moreover, in the spinal nerve ligation model, administration of A 438079 reduced the neuropathic pain induced by the application of a von Frey hair. The ED50 value was 76 μM/kg [2].
References:
1. Nelson D W, Gregg R J, Kort M E, et al. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. Journal of medicinal chemistry, 2006, 49(12): 3659-3666.
2. Donnelly-Roberts D L, Jarvis M F. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. British journal of pharmacology, 2007, 151(5): 571-579.
- Gymnoside III
Catalog No.:BCN8218
CAS No.:899430-03-6
- Beta-Carboline-1-propanoic acid
Catalog No.:BCN5805
CAS No.:89915-39-9
- 3-O-Acetyl-alpha-boswellic acid
Catalog No.:BCN2671
CAS No.:89913-60-0
- 3-Epichromolaenide
Catalog No.:BCN7241
CAS No.:89913-53-1
- 4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one hydrochloride
Catalog No.:BCC8647
CAS No.:898543-06-1
- XL228
Catalog No.:BCC2058
CAS No.:898280-07-4
- Esculentoside D
Catalog No.:BCN5013
CAS No.:89808-50-4
- YS-035 hydrochloride
Catalog No.:BCC6639
CAS No.:89805-39-0
- PF-3758309
Catalog No.:BCC1853
CAS No.:898044-15-0
- 5-Ethyltio-1H-Tetrazole
Catalog No.:BCC2844
CAS No.:89797-68-2
- Aceclofenac
Catalog No.:BCC5233
CAS No.:89796-99-6
- Myrianthic acid
Catalog No.:BCN7130
CAS No.:89786-84-5
- Platycoside K
Catalog No.:BCN3242
CAS No.:899447-64-4
- A 438079
Catalog No.:BCC1316
CAS No.:899507-36-9
- ML 348
Catalog No.:BCC5611
CAS No.:899713-86-1
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
- 3-Nitro-1-(4-octylphenyl)-1-propanone
Catalog No.:BCN2250
CAS No.:899822-97-0
- 3-(Hydroxymethyl)-3-nitro-1-(4-octylphenyl)-1,4-butanediol
Catalog No.:BCN1313
CAS No.:899822-99-2
- Salicyl alcohol
Catalog No.:BCN4442
CAS No.:90-01-7
- Guaiacol
Catalog No.:BCN8311
CAS No.:90-05-1
- 1-Naphthol
Catalog No.:BCC8473
CAS No.:90-15-3
- beta-Rhamnocitrin
Catalog No.:BCN3293
CAS No.:90-19-7
- Xanthoxylin
Catalog No.:BCN4443
CAS No.:90-24-4
- 4-Methylumbelliferone
Catalog No.:BCN2563
CAS No.:90-33-5
Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model.[Pubmed:21924325]
Neurosci Lett. 2011 Oct 17;504(1):57-61.
Peripheral nerve injury causes a progressive series of morphological changes in spinal microglia, and extracellular ATP stimulates proliferation of microglia and may be involved in neuropathic pain. We defined the precise expression of P2X7 in the spinal cord following peripheral nerve injury. We found that both P2X7 mRNA and protein increased in the spinal cord, with a peak at 7d after injury. Double labeling studies revealed that cells expressing increased P2X7 mRNA and protein after nerve injury were predominantly microglia in dorsal horn. Pharmacological blockades by intrathecal administration of a P2X7 antagonist (A 438079 hydrochloride) suppressed the development of mechanical hypersensitivity. We present distinct evidence that increases in the number of P2X7 receptors in spinal microglia may play an important role in neuropathic pain.
Subcellular distribution and early signalling events of P2X7 receptors from mouse cerebellar granule neurons.[Pubmed:25446427]
Eur J Pharmacol. 2014 Dec 5;744:190-202.
The subcellular distribution and early signalling events of P2X7 receptors were studied in mouse cerebellar granule neurons. Whole-cell patch-clamp recordings evidenced inwardly directed non-desensitizing currents following adenosine 5'-triphosphate (ATP; 600 microM) or 2'-3'-o-(4-benzoylbenzoyl)-adenosine 5'-triphosphate (BzATP; 100 microM) administration to cells bathed in a medium with no-added divalent cations (Ca(2+) and Mg(2+)). Nucleotide-activated currents were inhibited by superfusion of 2.5 mM Ca(2+), 1.2 mM Mg(2+) or 100 nM Brilliant Blue G (BBG), hence indicating the expression of ionotropic P2X7 receptors. Fura-2 calcium imaging showed [Ca(2+)]i elevations in response to ATP or BzATP at the somas and at a small number of axodendritic regions of granule neurons. Differential sensitivity of these [Ca(2+)]i increases to three different P2X7 receptor antagonists (100 nM BBG, 10 muM 4-[(2S)-2-[(5-isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl) propyl] phenyl isoquinolinesulfonic acid ester, KN-62, and 1 muM 3-(5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl)methyl pyridine hydrochloride hydrate, A-438079) revealed that P2X7 receptors are co-expressed with different P2Y receptors along the plasmalemma of granule neurons. Finally, experiments with the fluorescent dye YO-PRO-1 indicated that prolonged stimulation of P2X7 receptors does not lead to the opening of a membrane pore permeable to large cations. Altogether, our results emphasise the expression of functional P2X7 receptors at both the axodendritic and somatic levels in mouse cerebellar granule neurons, and favour the notion that P2X7 receptors might function in a subcellular localisation-specific manner: presynaptically, by controlling glutamate release, and on the cell somas, by supporting granule neuron survival against glutamate excytotoxicity.
Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states.[Pubmed:17471177]
Br J Pharmacol. 2007 Jul;151(5):571-9.
ATP-sensitive P2X(7) receptors are localized on cells of immunological origin including peripheral macrophages and glial cells in the CNS. Activation of P2X(7) receptors leads to rapid changes in intracellular calcium concentrations, release of the proinflammatory cytokine interleukin-1beta and following prolonged agonist exposure, the formation of cytolytic pores in plasma membranes. Both the localization and functional consequences of P2X(7) receptor activation indicate a role in inflammatory processes. The phenotype of P2X(7) receptor gene-disrupted mice also indicates that P2X(7) receptor activation contributes to ongoing inflammation. More recently, P2X(7) receptor knockout data has also suggested a specific role in inflammatory and neuropathic pain states. The recent discovery of potent and highly selective antagonists for P2X(7) receptors has helped to further clarify P2X receptor pharmacology, expanded understanding of P2X(7) receptor signaling, and offers new evidence that P2X(7) receptors play a specific role in nociceptive signaling in chronic pain states. In this review, we incorporate the recent discoveries of novel P2X(7) receptor-selective antagonists with a brief update on P2X(7) receptor pharmacology and its therapeutic potential.
P2X7-related modulation of pathological nociception in rats.[Pubmed:17478048]
Neuroscience. 2007 Jun 8;146(4):1817-28.
Growing evidence supports a role for the immune system in the induction and maintenance of chronic pain. ATP is a key neurotransmitter in this process. Recent studies demonstrate that the glial ATP receptor, P2X7, contributes to the modulation of pathological pain. To further delineate the endogenous mechanisms that are involved in P2X7-related antinociception, we utilized a selective P2X7 receptor antagonist, A-438079, in a series of in vivo and in vitro experiments. Injection of A-438079 (10-300 micromol/kg, i.p.) was anti-allodynic in three different rat models of neuropathic pain and it attenuated formalin-induced nocifensive behaviors. Using in vivo electrophysiology, A-438079 (80 micromol/kg, i.v.) reduced noxious and innocuous evoked activity of different classes of spinal neurons (low threshold, nociceptive specific, wide dynamic range) in neuropathic rats. The effects of A-438079 on evoked firing were diminished or absent in sham rats. Spontaneous activity of all classes of spinal neurons was also significantly reduced by A-438079 in neuropathic but not sham rats. In vitro, A-438079 (1 microM) blocked agonist-induced (2,3-O-(4-benzoylbenzoyl)-ATP, 30 microM) current in non-neuronal cells taken from the vicinity of the dorsal root ganglia. Furthermore, A-438079 dose-dependently (0.3-3 microM) decreased the quantity of the cytokine, interleukin-1beta, released from peripheral macrophages. Thus, ATP, acting through the P2X7 receptor, exerts a wide-ranging influence on spinal neuronal activity following a chronic injury. Antagonism of the P2X7 receptor can in turn modulate central sensitization and produce antinociception in animal models of pathological pain. These effects are likely mediated through immuno-neural interactions that affect the release of endogenous cytokines.
Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists.[Pubmed:16759108]
J Med Chem. 2006 Jun 15;49(12):3659-66.
1-Benzyl-5-aryltetrazoles were discovered to be novel antagonists for the P2X(7) receptor. Structure-activity relationship (SAR) studies were conducted around both the benzyl and phenyl moieties. In addition, the importance of the regiochemical substitution on the tetrazole was examined. Compounds were evaluated for activity to inhibit calcium flux in both human and rat recombinant P2X(7) cell lines using fluorometric imaging plate reader technology. Analogues were also assayed for their ability to inhibit IL-1beta release and to inhibit P2X(7)-mediated pore formation in human THP-1 cells. Compound 15d was advanced to efficacy studies in a model of neuropathic pain where significant reversal of mechanical allodynia was observed at doses that did not affect motor coordination.


