XACCAS# 96865-92-8 |
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- AG-1478
Catalog No.:BCC3717
CAS No.:153436-53-4
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Gefitinib hydrochloride
Catalog No.:BCC1591
CAS No.:184475-55-6
- Lapatinib Ditosylate
Catalog No.:BCC2083
CAS No.:388082-78-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 96865-92-8 | SDF | Download SDF |
| PubChem ID | 5697 | Appearance | Powder |
| Formula | C21H28N6O4 | M.Wt | 428.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Limited solubility | ||
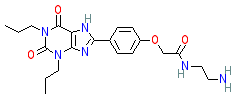
| Chemical Name | N-(2-aminoethyl)-2-[4-(2,6-dioxo-1,3-dipropyl-7H-purin-8-yl)phenoxy]acetamide | ||
| SMILES | CCCN1C2=C(C(=O)N(C1=O)CCC)NC(=N2)C3=CC=C(C=C3)OCC(=O)NCCN | ||
| Standard InChIKey | FIQGIOAELHTLHM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

XAC Dilution Calculator

XAC Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- XCC
Catalog No.:BCC7890
CAS No.:96865-83-7
- Maoecrystal A
Catalog No.:BCN5407
CAS No.:96850-30-5
- Maoecrystal B
Catalog No.:BCN4516
CAS No.:96850-29-2
- 8beta-Tigloyloxycostunolide
Catalog No.:BCN7115
CAS No.:96850-21-4
- Indatraline hydrochloride
Catalog No.:BCC7123
CAS No.:96850-13-4
- Przewaquinone C
Catalog No.:BCN3003
CAS No.:96839-29-1
- Orlistat
Catalog No.:BCC3830
CAS No.:96829-58-2
- Chlorovaltrate K
Catalog No.:BCN7126
CAS No.:96801-92-2
- (±)-McN 5652
Catalog No.:BCC7267
CAS No.:96795-89-0
- Chlorisondamine diiodide
Catalog No.:BCC6885
CAS No.:96750-66-2
- [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-Substance P
Catalog No.:BCC7211
CAS No.:96736-12-8
- Rabdoserrin A
Catalog No.:BCN8041
CAS No.:96685-01-7
- VIP (guinea pig)
Catalog No.:BCC5725
CAS No.:96886-24-7
- Nyasol
Catalog No.:BCN7579
CAS No.:96895-25-9
- 1,2-Bis(4'-methyl-2,2'-bipyridin-4-yl)ethane
Catalog No.:BCC8414
CAS No.:96897-04-0
- Cyproheptadine hydrochloride
Catalog No.:BCC5161
CAS No.:969-33-5
- Artanin
Catalog No.:BCN4517
CAS No.:96917-26-9
- Cisatracurium Besylate
Catalog No.:BCC4345
CAS No.:96946-42-8
- Momordin Ic
Catalog No.:BCN1216
CAS No.:96990-18-0
- DTG
Catalog No.:BCC6812
CAS No.:97-39-2
- Eugenol
Catalog No.:BCN5964
CAS No.:97-53-0
- Isoeugenol
Catalog No.:BCN8312
CAS No.:97-54-1
- Solvent Yellow 3
Catalog No.:BCC9150
CAS No.:97-56-3
- Allantoin
Catalog No.:BCN4527
CAS No.:97-59-6
Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine.[Pubmed:21885291]
Structure. 2011 Sep 7;19(9):1283-93.
Methylxanthines, including caffeine and theophylline, are among the most widely consumed stimulant drugs in the world. These effects are mediated primarily via blockade of adenosine receptors. Xanthine analogs with improved properties have been developed as potential treatments for diseases such as Parkinson's disease. Here we report the structures of a thermostabilized adenosine A(2A) receptor in complex with the xanthines xanthine amine congener and caffeine, as well as the A(2A) selective inverse agonist ZM241385. The receptor is crystallized in the inactive state conformation as defined by the presence of a salt bridge known as the ionic lock. The complete third intracellular loop, responsible for G protein coupling, is visible consisting of extended helices 5 and 6. The structures provide new insight into the features that define the ligand binding pocket of the adenosine receptor for ligands of diverse chemotypes as well as the cytoplasmic regions that interact with signal transduction proteins.
[(3)H]XAC (xanthine amine congener) is a radioligand for A(2)-adenosine receptors in rabbit striatum.[Pubmed:20504695]
Neurochem Int. 1991;18(2):207-13.
The intrinsic affinity of 8-phenylxanthine analogs at striatal A(2)-adenosine receptors is highly species dependent. [(3)H]XAC (8-[2-aminoethyl[amino[carbonyl[methyl[oxyphenyl]]]]]-1,3-dipropylxanthine), although A(1)-selective in the rat brain, binds to A(2) receptors in rabbit striatal membranes with sufficiently high affinity to serve as a radioligand. In the presence of 50 nM CPX (8-cyclopentyl-1,3-dipropylxanthine), an A(1)-selective antagonist added to eliminate binding to A(1) receptors, [(3)H]XAC exhibits saturable, specific binding (70% of total) to A(2) sites with a K(d) of 3.8 nM and a B(max) of 1.23 pmol/mg protein. At 24 degrees C, the association and dissociation rate constants were 0.13 min(?1) nM(?1) and 0.36 min(?1), respectively. Binding was performed for 1 h, with non-specific binding defined in the presence of 100 ?M NECA (N-ethylcarboxamidoadenosine). The potency order for antagonists against 1 nM [(3)H]XAC at rabbit A(2)-receptors was XAC ? N(?)-Me-XAC ? CPX = XCC > 1,3-dipropyl-8-p-sulfophenylxanthine > PSPT. The relative potency order for agonists was CGS ? NECA > APEC [= 2-(aminoethylaminocarbonyl-ethylphenylethylamino)-NECA] > PAPA-APEC > ADAC > R-PIA (N(6)-phenylisopropyladenosine) > S-PIA. The ability to characterize central A(2)-adenosine receptors using an antagonist ligand that is chemically functionalized offers the possibility to design affinity labeling probes for this receptor subtype in the brain, similar to those antagonist probes already developed for A(1)-receptors. The results also suggest that affinity columns containing chemically immobilized XAC may be used for isolating central A(2)-adenosine receptors from rabbit striatum.
Xenopus actin depolymerizing factor/cofilin (XAC) is responsible for the turnover of actin filaments in Listeria monocytogenes tails.[Pubmed:9087446]
J Cell Biol. 1997 Mar 24;136(6):1323-32.
In contrast to the slow rate of depolymerization of pure actin in vitro, populations of actin filaments in vivo turn over rapidly. Therefore, the rate of actin depolymerization must be accelerated by one or more factors in the cell. Since the actin dynamics in Listeria monocytogenes tails bear many similarities to those in the lamellipodia of moving cells, we have used Listeria as a model system to isolate factors required for regulating the rapid actin filament turnover involved in cell migration. Using a cell-free Xenopus egg extract system to reproduce the Listeria movement seen in a cell, we depleted candidate depolymerizing proteins and analyzed the effect that their removal had on the morphology of Listeria tails. Immunodepletion of Xenopus actin depolymerizing factor (ADF)/cofilin (XAC) from Xenopus egg extracts resulted in Listeria tails that were approximately five times longer than the tails from undepleted extracts. Depletion of XAC did not affect the tail assembly rate, suggesting that the increased tail length was caused by an inhibition of actin filament depolymerization. Immunodepletion of Xenopus gelsolin had no effect on either tail length or assembly rate. Addition of recombinant wild-type XAC or chick ADF protein to XAC-depleted extracts restored the tail length to that of control extracts, while addition of mutant ADF S3E that mimics the phosphorylated, inactive form of ADF did not reduce the tail length. Addition of excess wild-type XAC to Xenopus egg extracts reduced the length of Listeria tails to a limited extent. These observations show that XAC but not gelsolin is essential for depolymerizing actin filaments that rapidly turn over in Xenopus extracts. We also show that while the depolymerizing activities of XAC and Xenopus extract are effective at depolymerizing normal filaments containing ADP, they are unable to completely depolymerize actin filaments containing AMPPNP, a slowly hydrolyzible ATP analog. This observation suggests that the substrate for XAC is the ADP-bound subunit of actin and that the lifetime of a filament is controlled by its nucleotide content.
Potent convulsant actions of the adenosine receptor antagonist, xanthine amine congener (XAC).[Pubmed:2779359]
Life Sci. 1989;45(8):719-28.
The convulsant properties of xanthine amine congener (XAC, 8-(4-(2-aminoethyl)-aminocarboxylmethyloxy)phenyl-1,3-dipropylxant hine) are compared to those of caffeine. Male Swiss albino mice were infused with convulsants through a lateral tail vein. Convulsion thresholds (i.e. the amount of convulsants required to elicit convulsions) of 39.8 +/- 2.0 mg/kg (n = 10) and 109.8 +/- 2.3 mg/kg (n = 10) were calculated for XAC and caffeine respectively. Pretreatment of animals with the adenosine receptor agonists 2-chloroadenosine, N6-cyclohexyladenosine or 5'-N-ethylcarboxamido-adenosine (1 mg/kg, i.p., 20 minutes prior to infusion) significantly decreased the seizure threshold of both XAC and caffeine. The adenosine uptake blockers, 6-nitrobenzylthioinosine or dipyridamole (0.25 mg/kg, i.p., 20 minutes prior to infusion) did not significantly affect the seizure threshold to either XAC or caffeine. The benzodiazepine agonist diazepam (5 mg/kg, i.p., 20 minutes prior to infusion) significantly increased the seizure threshold to both XAC (p less than 0.05) and caffeine (p less than 0.01), whereas the benzodiazepine antagonist Ro 15-1788 (10 mg/kg, i.p., 20 minutes prior to infusion) significantly increased the seizure threshold to caffeine (p less than 0.01), but not XAC. The results suggest that actions at benzodiazepine receptors may be a tenable hypothesis to explain the convulsant actions of caffeine, but not those of XAC.


