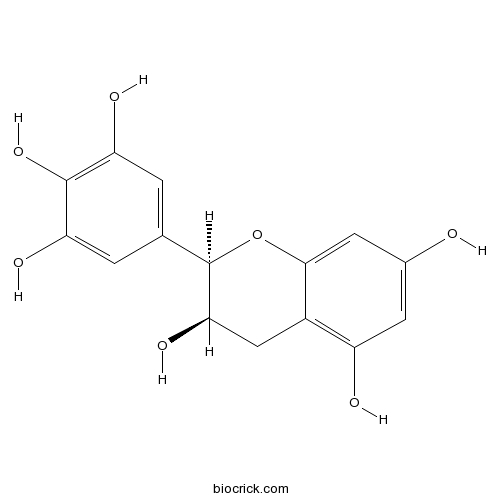
(-)-Epigallocatechin(EGC)green tea epicatechin CAS# 970-74-1 |
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 970-74-1 | SDF | Download SDF |
| PubChem ID | 72277 | Appearance | White-light ochre powder |
| Formula | C15H14O7 | M.Wt | 306.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | EGC; Epigallocatechin; l-Epigallocatechin; epi-Gallocatechin | ||
| Solubility | DMSO : ≥ 150 mg/mL (489.76 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2R,3R)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | ||
| SMILES | C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C(=C3)O)O)O)O | ||
| Standard InChIKey | XMOCLSLCDHWDHP-IUODEOHRSA-N | ||
| Standard InChI | InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (-)-Epigallocatechin exhibits antiplatelet,anticoagulation,anti-tumor,anti-cancer and anti-inflammatory functions. Epigallocatechin activates haem oxygenase-1 expression via protein kinase Cdelta and Nrf2. Cu(2+) with (-)-epigallocatechin (EGC) facilitated DNA cleavage, while Ag+ with EGC showed a strong repressive effect. |
| Targets | LDL | NO | HO-1 |
| In vitro | Isolation and characterization of rat intestinal bacteria involved in biotransformation of (-)-epigallocatechin.[Pubmed: 24947740]Arch Microbiol. 2014 Oct;196(10):681-95.Two intestinal bacterial strains MT4s-5 and MT42 involved in the degradation of (-)-Epigallocatechin(EGC) were isolated from rat feces. Green tea catechins suh α(–)-epicatechin and (–)-epigallocatechin accelerate Cu2+-induced low density lipoprotein in propagation phase[Reference: WebLink]Febs Letters, 1997, 401(2-3):230-234.
DNA cleavage activities of (-)-epigallocatechin, (-)-epicatechin, (+)-catechin, and (-)-epigallocatechin gallate with various kinds of metal ions.[Pubmed: 10610127]Biosci Biotechnol Biochem. 1999 Sep;63(9):1654-6.The DNA cleavage activities of (+)-catechin (C), (-)-epicatechin (EC), (-)-Epigallocatechin(EGC), and (-)-epigallocatechin gallate (EGCg) were examined with 16 different metal ions. Cu(2+) with all the catechins facilitated DNA cleavage, while Ag+ with EGC and EC showed a strong repressive effect. The other metal ions examined showed little effect. |
| In vivo | Blood anticoagulation and antiplatelet activity of green tea (-)-epigallocatechin (EGC) in mice.[Pubmed: 24056410]Food Funct. 2013 Oct;4(10):1521-5.(-)-Epigallocatechin(EGC) was prepared from green tea polyphenols through column chromatography of a polyamide (3.6 × 40 cm). |

(-)-Epigallocatechin(EGC) Dilution Calculator

(-)-Epigallocatechin(EGC) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2648 mL | 16.3239 mL | 32.6477 mL | 65.2955 mL | 81.6193 mL |
| 5 mM | 0.653 mL | 3.2648 mL | 6.5295 mL | 13.0591 mL | 16.3239 mL |
| 10 mM | 0.3265 mL | 1.6324 mL | 3.2648 mL | 6.5295 mL | 8.1619 mL |
| 50 mM | 0.0653 mL | 0.3265 mL | 0.653 mL | 1.3059 mL | 1.6324 mL |
| 100 mM | 0.0326 mL | 0.1632 mL | 0.3265 mL | 0.653 mL | 0.8162 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(-)-epigallocatechin is a polyphenol in green tea [1].
(-)-epigallocatechin (EGC) plays an important role in cell growth inhibition, apoptosis and bone metabolism.
(-)-epigallocatechin is a polyphenol in green tea. In MCF-7 and MDA-MB-231 breast cancer cell lines, EGC significantly inhibited cell growth and induced apoptosis, which were independent of p53 and required Fas signaling [1]. In H1299 cells, EGC (10-40 µM) reduced cell viability in a dose dependent way. In Hep-3B cells, EGC (40/80 µM) also reduced cell viability. However, EGC (20 µM) slightly increased cell viabilities in SK-Hep-1 and OECM-1 cells but significantly decreased cell viabilities at concentrations up to 40 µM. EGC exhibited cytotoxicity with IC50 values of 26, 33 and 22 µM in H1299, OECM-1 and SAS cells, respectively. In H1299, OECM-1 and SAS cells, EGC (40 µM) induced apoptosis by 30, 28 and 24%, respectively. In H1299 cells, EGC (40 µM) inhibited hTERT promoter activity in a time- and dose- dependent way and also inhibited hTERT mRNA expression, which was important for cell proliferation [2].
In ICR mice, EGC (0.5 and 1.0 g/kg/d) significantly inhibited platelet aggregation by 18.4% and 25.6% respectively and also significantly prolonged blood clotting time (BCT) and bleeding time (BT), which suggested that the blood anticoagulation and antiplatelet activity of EGC [3].
References:
[1]. Vergote D, Cren-Olivé C, Chopin V, et al. (-)-Epigallocatechin (EGC) of green tea induces apoptosis of human breast cancer cells but not of their normal counterparts. Breast Cancer Res Treat, 2002, 76(3): 195-201.
[2]. Lin SC, Li WC, Shih JW, et al. The tea polyphenols EGCG and EGC repress mRNA expression of human telomerase reverse transcriptase (hTERT) in carcinoma cells. Cancer Lett, 2006, 236(1): 80-88.
[3]. Chen XQ, Wang XB, Guan RF, et al. Blood anticoagulation and antiplatelet activity of green tea (-)-epigallocatechin (EGC) in mice. Food Funct, 2013, 4(10): 1521-1525.
- (+)-Gallocatechin
Catalog No.:BCN5928
CAS No.:970-73-0
- Disulfiram
Catalog No.:BCC2098
CAS No.:97-77-8
- Ac-Ala-OH
Catalog No.:BCC3189
CAS No.:97-69-8
- 2-Methylvaleric acid
Catalog No.:BCN8498
CAS No.:97-61-0
- Allantoin
Catalog No.:BCN4527
CAS No.:97-59-6
- Solvent Yellow 3
Catalog No.:BCC9150
CAS No.:97-56-3
- Isoeugenol
Catalog No.:BCN8312
CAS No.:97-54-1
- Eugenol
Catalog No.:BCN5964
CAS No.:97-53-0
- DTG
Catalog No.:BCC6812
CAS No.:97-39-2
- Momordin Ic
Catalog No.:BCN1216
CAS No.:96990-18-0
- Cisatracurium Besylate
Catalog No.:BCC4345
CAS No.:96946-42-8
- Artanin
Catalog No.:BCN4517
CAS No.:96917-26-9
- Porfimer Sodium
Catalog No.:BCC5353
CAS No.:97067-70-4
- AMN 082 dihydrochloride
Catalog No.:BCC7344
CAS No.:97075-46-2
- 6-Geranylnaringenin
Catalog No.:BCN3001
CAS No.:97126-57-3
- 6-Epi-8-O-acetylharpagide
Catalog No.:BCN4550
CAS No.:97169-44-3
- 3-Ethoxyandrosta-3,5-dien-17-one
Catalog No.:BCC8630
CAS No.:972-46-3
- Meisoindigo
Catalog No.:BCC5132
CAS No.:97207-47-1
- Eriobofuran
Catalog No.:BCN7436
CAS No.:97218-06-9
- Picfeltarraenin IB
Catalog No.:BCN2845
CAS No.:97230-46-1
- Picfeltarraenin IA
Catalog No.:BCN1041
CAS No.:97230-47-2
- Topiramate
Catalog No.:BCC2314
CAS No.:97240-79-4
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
- 8-Hydroxy-4-cadinen-3-one
Catalog No.:BCN4520
CAS No.:97372-53-7
DNA cleavage activities of (-)-epigallocatechin, (-)-epicatechin, (+)-catechin, and (-)-epigallocatechin gallate with various kinds of metal ions.[Pubmed:10610127]
Biosci Biotechnol Biochem. 1999 Sep;63(9):1654-6.
The DNA cleavage activities of (+)-catechin (C), (-)-epicatechin (EC), (-)-epigallocatechin (EGC), and (-)-epigallocatechin gallate (EGCg) were examined with 16 different metal ions. Cu(2+) with all the catechins facilitated DNA cleavage, while Ag+ with EGC and EC showed a strong repressive effect. The other metal ions examined showed little effect.
Isolation and characterization of rat intestinal bacteria involved in biotransformation of (-)-epigallocatechin.[Pubmed:24947740]
Arch Microbiol. 2014 Oct;196(10):681-95.
Two intestinal bacterial strains MT4s-5 and MT42 involved in the degradation of (-)-epigallocatechin (EGC) were isolated from rat feces. Strain MT4s-5 was tentatively identified as Adlercreutzia equolifaciens. This strain converted EGC into not only 1-(3, 4, 5-trihydroxyphenyl)-3-(2, 4, 6-trihydroxyphenyl)propan-2-ol (1), but also 1-(3, 5-dihydroxyphenyl)-3-(2, 4, 6-trihydroxyphenyl)propan-2-ol (2), and 4'-dehydroxylated EGC (7). Type strain (JCM 9979) of Eggerthella lenta was also found to convert EGC into 1. Strain MT42 was identified as Flavonifractor plautii and converted 1 into 4-hydroxy-5-(3, 4, 5-trihydroxyphenyl)valeric acid (3) and 5-(3, 4, 5-trihydroxyphenyl)-gamma-valerolactone (4) simultaneously. Strain MT42 also converted 2 into 4-hydroxy-5-(3, 5-dihydroxyphenyl)valeric acid (5), and 5-(3, 5-dihydroxyphenyl)-gamma-valerolactone (6). Furthermore, F. plautii strains ATCC 29863 and ATCC 49531 were found to catalyze the same reactions as strain MT42. Interestingly, formation of 2 from EGC by strain MT4s-5 occurred rapidly in the presence of hydrogen supplied by syntrophic bacteria. Strain JCM 9979 also formed 2 in the presence of the hydrogen or formate. Strain MT4s-5 converted 1, 3, and 4 to 2, 5, and 6, respectively, and the conversion was stimulated by hydrogen, whereas strain JCM 9979 could catalyze the conversion only in the presence of hydrogen or formate. On the basis of the above results together with previous reports, the principal metabolic pathway of EGC and EGCg by catechin-degrading bacteria in gut tract is proposed.
Blood anticoagulation and antiplatelet activity of green tea (-)-epigallocatechin (EGC) in mice.[Pubmed:24056410]
Food Funct. 2013 Oct;4(10):1521-5.
EGC was prepared from green tea polyphenols through column chromatography of a polyamide (3.6 x 40 cm). Three dosages of EGC (0.25, 0.5, 1.0 g kg(-1) d(-1)) were ingested respectively by ICR mice via gavage. Compared with the control group, group EGC0.5 (dosage, 0. 5 g kg(-1) d(-1)) and group EGC1.0 (dosage, 1.0 g kg(-1) d(-1)) presented significant inhibition on platelet aggregation in mice accompanied by 18.4 and 25.6% of inhibition ratio, respectively. The bleeding times (BT) of mice in group EGC0.5 and group EGC1.0 were significantly prolonged (P < 0.01) as well as blood clotting time (BCT) in group EGC1.0 (P < 0.05). All three dosages of EGC prolonged activated partial thromboplastin time (APTT) significantly (P < 0.01), but had no prominent effect on prothrombin time (PT) and fibrinogen level which indicated that the anticoagulation of EGC could not be attributed to the level decrease of coagulation factor such as fibrinogen. The results demonstrated that EGC had prominent antiplatelet activity and blood anticoagulation in a dose-dependent manner.


