PalosuranUrotensin-II receptor antagonist CAS# 540769-28-6 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 540769-28-6 | SDF | Download SDF |
| PubChem ID | 10173280 | Appearance | Powder |
| Formula | C25H30N4O2 | M.Wt | 418.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ACT-058362 | ||
| Solubility | DMSO : ≥ 30 mg/mL (71.68 mM) *"≥" means soluble, but saturation unknown. | ||
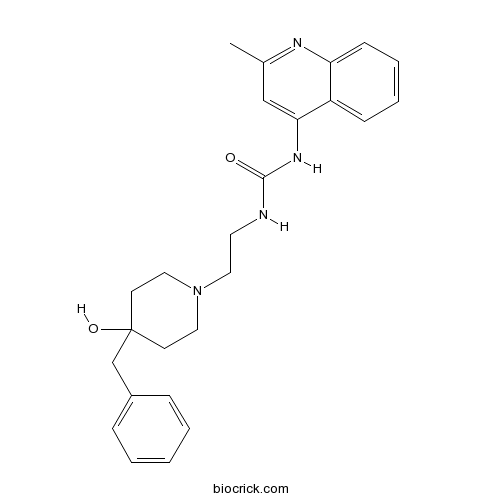
| Chemical Name | 1-[2-(4-benzyl-4-hydroxypiperidin-1-yl)ethyl]-3-(2-methylquinolin-4-yl)urea | ||
| SMILES | CC1=NC2=CC=CC=C2C(=C1)NC(=O)NCCN3CCC(CC3)(CC4=CC=CC=C4)O | ||
| Standard InChIKey | WYJCYXOCHXWTHG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H30N4O2/c1-19-17-23(21-9-5-6-10-22(21)27-19)28-24(30)26-13-16-29-14-11-25(31,12-15-29)18-20-7-3-2-4-8-20/h2-10,17,31H,11-16,18H2,1H3,(H2,26,27,28,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Palosuran (ACT-058362) is a new potent and specific antagonist of the human UT receptor with an IC50 of 3.6±0.2 nM.
IC50 Value: 3.6±0.2 nM [1]
Target: Urotensin Receptor (GPR14)
in vitro: Palosuran inhibited 125I-U-II binding to human UT receptors in membrane preparations from CHO cells carrying the human UT receptors almost as potently as cold U-II, with an IC50 of 3.6 ± 0.2 nM. On cells, the inhibitory binding potency of palosuran against human UT receptor was lower than on membranes (IC50 = 46.2 ± 13 nM on TE 671 cells and 86 ± 30 nM on recombinant CHO cells). Compared with the human UT receptor, the binding inhibitory potency of palosuran against the rat UT receptor was lower in membrane preparation (400-fold), as well as in cells (>120-fold) [1].
in vivo: Long-term treatment of streptozotocin-induced diabetic rats with palosuran improved survival, increased insulin, and slowed the increase in glycemia, glycosylated hemoglobin, and serum lipids. Furthermore, palosuran increased renal blood flow and delayed the development of proteinuria and renal damage [2]. Palosuran was rapidly absorbed with maximum plasma concentrations at 1 hour after drug administration. The accumulation factor was 1.7 (geometric mean) (95% confidence interval, 1.3 to 2.1).Palosuran was well tolerated [3]. In mesenteric vessels, palosuran treatment up-regulated expression of RhoA and Rho-kinase, increased Rho-kinase-activity, and diminished nitric oxide (NO)/cyclic guanosine 3',5'-monophosphate (cGMP) signaling. Moreover, palosuran increased renal blood flow, sodium, and water excretion in BDL rats [4].
Toxicity: Palosuran was well tolerated. No serious adverse events or dose-related adverse events were reported. No treatment-related pattern was detected for vital signs, clinical laboratory parameters, or electrocardiography parameters [5]. References: | |||||

Palosuran Dilution Calculator

Palosuran Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3893 mL | 11.9466 mL | 23.8931 mL | 47.7863 mL | 59.7329 mL |
| 5 mM | 0.4779 mL | 2.3893 mL | 4.7786 mL | 9.5573 mL | 11.9466 mL |
| 10 mM | 0.2389 mL | 1.1947 mL | 2.3893 mL | 4.7786 mL | 5.9733 mL |
| 50 mM | 0.0478 mL | 0.2389 mL | 0.4779 mL | 0.9557 mL | 1.1947 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.2389 mL | 0.4779 mL | 0.5973 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Palosuran is a potent and specific antagonist of human urotensin-II receptor with IC50 value of 3.6nM [1].
Palosuran is a nonpeptidic, potent and selective antagonist of the urotensin-II receptor. It is selective toward human receptor. The IC50 value of it to rat receptor is 1475nM. The efficacy of palosuran to inhibit Ca2+ mobilization in CHO cells also improved that palosuran is more effective to human UT receptors than to rat UT receptors with IC50 values of 17nM and more than 10μM, respectively. Besides that, palosuran inhibits the phosphorylation of MAPK with IC50 value of 150nM in recombinant CHO cells. Palosuran is selective. It shows no inhibition of other receptors involved in vascular tone regulation such as α-1 adrenoceptor, 5-hydroxytryptamine 2A receptor and endothelin receptor A. Palosuran is used as a pharmacological tool to study the role of endogenous U-II [1, 2].
References:
[1] Carotenuto A, Grieco P, Rovero P, et al. Urotensin-II receptor antagonists. Current medicinal chemistry, 2006, 13(3): 267-275.
[2] Clozel M, Binkert C, Birker-Robaczewska M, et al. Pharmacology of the urotensin-II receptor antagonist palosuran (ACT-058362; 1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulfate salt): first demonstration of a pathophysiological role of the urotensin system. Journal of Pharmacology and Experimental Therapeutics, 2004, 311(1): 204-212.
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
- Etonogestrel
Catalog No.:BCC5230
CAS No.:54048-10-1
- Albendazole Oxide
Catalog No.:BCC4757
CAS No.:54029-12-8
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Pentylenetetrazole
Catalog No.:BCC7453
CAS No.:54-95-5
- Isoniazid
Catalog No.:BCC9003
CAS No.:54-85-3
- Cinanserin hydrochloride
Catalog No.:BCC6653
CAS No.:54-84-2
- Pilocarpine HCl
Catalog No.:BCC4702
CAS No.:54-71-7
- Idoxuridine
Catalog No.:BCC4666
CAS No.:54-42-2
- Metyrapone
Catalog No.:BCC7632
CAS No.:54-36-4
- Furosemide
Catalog No.:BCC3782
CAS No.:54-31-9
- Sodium salicylate
Catalog No.:BCC4846
CAS No.:54-21-7
- Isoastilbin
Catalog No.:BCN5719
CAS No.:54081-48-0
- 2-(1-Hydroxy-1-methylethyl)-4-methoxy-7H-furo[3,2-g][1]benzopyran-7-one
Catalog No.:BCN1422
CAS No.:54087-32-0
- L-Carnitine inner salt
Catalog No.:BCN1229
CAS No.:541-15-1
- Decamethonium Bromide
Catalog No.:BCC4568
CAS No.:541-22-0
- Isovaleramide
Catalog No.:BCC4668
CAS No.:541-46-8
- Muscone
Catalog No.:BCN6275
CAS No.:541-91-3
- 15-Hydroxydehydroabietic acid
Catalog No.:BCN5720
CAS No.:54113-95-0
- 9-Benzylcarbazole-3-carboxaldehyde
Catalog No.:BCC8800
CAS No.:54117-37-2
- Apoptosis Inhibitor
Catalog No.:BCC1143
CAS No.:54135-60-3
- Neoisoastilbin
Catalog No.:BCN6532
CAS No.:54141-72-9
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Vicriviroc Malate
Catalog No.:BCC1230
CAS No.:541503-81-5
Effectiveness of palosuran in bleomycin-induced experimental scleroderma.[Pubmed:22886350]
Inflammation. 2013 Feb;36(1):75-9.
Systemic sclerosis (SSc) is a disease characterized by skin and internal organ involvement. There is progressive accumulation of extracellular matrix components in the skin and involved organs. Tissue fibrosis is the prominent reason for mortality, and still, there is no satisfactory treatment. The aim of this study was to evaluate the effects of urotensin-II (U-II) antagonist Palosuran in an animal model of scleroderma. We also planned to measure U-II, endothelin-1 (ET-1), and transforming growth factor-beta1 (TGF-beta1) levels, as well as the association of these levels with dermal thickness. Twenty-four male mice were included in this study and they were divided into three groups--group 1: control group, group 2: fibrosis group, and group 3: fibrosis + Palosuran treatment group. Fibrosis + Palosuran treatment in group 3 reduced ET-1, U-II, and TGF-beta1 levels. In total, the diminished values were statistically significant in the ET-1 and TGF-beta1 levels (p < 0.05). Dermal thickness was higher in the fibrosis group, when compared with the other groups. There was no significant relationship between dermal thickness and ET-1, U-II, or TGF-beta1 levels (p > 0.05). It is believed that U-II is an important mediator in SSc, and its antagonism with Palosuran could be a new treatment choice in SSc.
Urotensin inhibition with palosuran could be a promising alternative in pulmonary arterial hypertension.[Pubmed:23100033]
Inflammation. 2013 Apr;36(2):405-12.
Pulmonary arterial hypertension (PAH) is a progressive and a life-threatening disease with its high morbidity and mortality ratios. On searching for new shining targets in pathogenesis, we noticed, in our previous studies, urotensin-II (UII) in systemic sclerosis with potent angiogenic and pro-fibrotic features. Owing to the mimicking properties of UII with endothelin-1 (ET1), we attempted to investigate the effect of Palosuran in a PAH rat model. Thirty rats were randomly divided into three groups, with each group comprising 10 rats: group 1 (control group) received the vehicle subcutaneously, instead of monocrotaline (MCT) and vehicle; group 2 (MCT group) received subcutaneous MCT and vehicle; and group 3 (MCT + Palosuran group) received subcutaneous MCT and Palosuran. Serum UII, ET1, transforming growth factor-beta1 (TGF-beta1) levels, pulmonary arteriolar pathology of different diameter vessels, and cardiac indices were evaluated. The ET1, TGF-beta1, and UII levels were significantly diminished in the treatment group, similar to the controls (p < 0.001). Right ventricular hypertrophy index and mean pulmonary arterial pressure scores were also significantly reduced in the treatment group (p = 0.001). Finally, in the 50-125-mum diameter arterioles, in contrast to Groups 3 and 1, there was a statistically significant thickness (p < 0.01) in the arteriolar walls of rats in Group 2. The treatment effect on arteries of more than 125-mum diameters was found to be valuable but not significant. Owing to its healing effect on hemodynamic, histological, and biochemical parameters of MCT-induced PAH, Palosuran as an antagonist of UII might be an optional treatment alternative for PAH.
Effect of the urotensin receptor antagonist palosuran in hypertensive patients with type 2 diabetic nephropathy.[Pubmed:20231521]
Hypertension. 2010 May;55(5):1206-9.
The urotensin system has been hypothesized to play an important role in the pathophysiology of diabetic nephropathy. In this multicenter, randomized, double-blind, placebo-controlled, 2-period crossover study, the effects of the urotensin receptor antagonist Palosuran on urinary albumin excretion and blood pressure in hypertensive patients with type 2 diabetic nephropathy treated with a single blocker of the renin-angiotensin-aldosterone system were assessed. Patients with 24-hour albuminuria >0.5 and <3.0 g, systolic blood pressure >135 and <170 mm Hg, and/or diastolic blood pressure >85 and <110 mm Hg received both Palosuran 125 mg BID and placebo for 4 weeks each. Fifty-four patients (20% women; mean age: 61.6 years, blood pressure: 155/84 mm Hg, and albuminuria: 1016 mg per 24 hours) were included in the per-protocol analysis. Palosuran did not affect albuminuria, blood pressure, glomerular filtration rate, or renal plasma flow significantly. These results question whether urotensin receptor antagonism represents a new treatment strategy in this high-risk patient population.
Palosuran treatment effective as bosentan in the treatment model of pulmonary arterial hypertension.[Pubmed:24604341]
Inflammation. 2014 Aug;37(4):1280-8.
Pulmonary arterial hypertension (PAH) is a progressive and fatal disorder that any valuable advance in the management of diseases has crucial importance. The present study aimed to compare the Endothelin1 (ET1) inhibitor bosentan which is regarded as standard therapy with different dose regimens of Palosuran which is urotensin-II (UII) inhibitor and explore the discrepancy for mean pulmonary arterial pressure (mPAP), UII, ET1 levels, and pulmonary vascular pathology. Seventy rats were randomly divided into seven groups of ten animals each: group 1 (control group) received the vehicle subcutaneously, instead of monocrotaline (MCT) and vehicle; group 2 (MCT group) received subcutaneous MCT and vehicle; and group 3 (MCT + Palosuran 30 mg) received subcutaneous MCT and Palosuran. Other groups consist of group 4 (MCT + Palosuran 100 mg), group 5 (MCT + bosentan 30 mg), group 6 (MCT + bosentan 100 mg), and group 7 (combination therapy). Serum ET1, UII, mPAP levels, and pulmonary arteriolar pathology of different diameter vessels of all groups have been measured and recorded. The ET1 and UII levels of untreated rats (group 2) were significantly higher than the other groups (p < 0.05). Moreover, mPAP levels of group 2 were significantly higher than the other groups (p = 0.001). Finally, 50-125-mum diameter of arteriole wall thickness was found to be significantly thicker in monocrotaline group compared to groups 4 and 6 (p < 0.001). Statistical differences of wall thickness/diameter ratios of arteries and arterioles larger than 125 was found to be significant between group 5, group 6, and the control group (p < 0.001). UII inhibitor is at least as effective as standard therapy bosentan. Findings of this study consolidate that Palosuran could be a new future promising therapeutic option in PAH.


