MonomethylsulochrinCAS# 10056-14-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10056-14-1 | SDF | Download SDF |
| PubChem ID | 23872041 | Appearance | Powder |
| Formula | C18H18O7 | M.Wt | 346.33 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
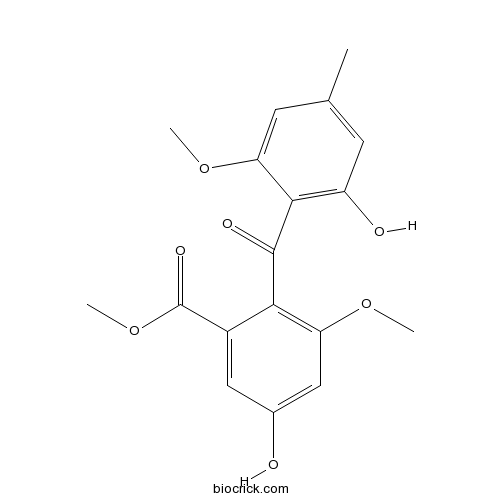
| Chemical Name | methyl 5-hydroxy-2-(2-hydroxy-6-methoxy-4-methylbenzoyl)-3-methoxybenzoate | ||
| SMILES | CC1=CC(=C(C(=C1)OC)C(=O)C2=C(C=C(C=C2C(=O)OC)O)OC)O | ||
| Standard InChIKey | XJOBKBUGVMLSEJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H18O7/c1-9-5-12(20)16(13(6-9)23-2)17(21)15-11(18(22)25-4)7-10(19)8-14(15)24-3/h5-8,19-20H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Monomethylsulochrin shows antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Staphylococcus aureus. 2. Monomethylsulochrin shows moderately inhibitory effects on the human bacterial pathogen Helicobacter pylori with the MIC value of 28.9+/-0.1 microM. |
| Targets | Antifection |

Monomethylsulochrin Dilution Calculator

Monomethylsulochrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8874 mL | 14.4371 mL | 28.8742 mL | 57.7484 mL | 72.1855 mL |
| 5 mM | 0.5775 mL | 2.8874 mL | 5.7748 mL | 11.5497 mL | 14.4371 mL |
| 10 mM | 0.2887 mL | 1.4437 mL | 2.8874 mL | 5.7748 mL | 7.2185 mL |
| 50 mM | 0.0577 mL | 0.2887 mL | 0.5775 mL | 1.155 mL | 1.4437 mL |
| 100 mM | 0.0289 mL | 0.1444 mL | 0.2887 mL | 0.5775 mL | 0.7219 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- LCL161
Catalog No.:BCC1691
CAS No.:1005342-46-0
- CVT 10216
Catalog No.:BCC5606
CAS No.:1005334-57-5
- Gelomulide N
Catalog No.:BCN6641
CAS No.:1005212-02-1
- Aeruginolactone
Catalog No.:BCN3695
CAS No.:1005208-88-7
- TCS 2002
Catalog No.:BCC6074
CAS No.:1005201-24-0
- Blasticidin A
Catalog No.:BCN1830
CAS No.:100513-53-9
- Sterigmatocystin
Catalog No.:BCN6885
CAS No.:10048-13-2
- Gastrin I (human)
Catalog No.:BCC5958
CAS No.:10047-33-3
- Rosiridin
Catalog No.:BCN5970
CAS No.:100462-37-1
- FFN 511
Catalog No.:BCC7799
CAS No.:1004548-96-2
- 1-EBIO
Catalog No.:BCC6904
CAS No.:10045-45-1
- TC ASK 10
Catalog No.:BCC6301
CAS No.:1005775-56-3
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- NF 546
Catalog No.:BCC7804
CAS No.:1006028-37-0
- MK-2894
Catalog No.:BCC1757
CAS No.:1006036-87-8
- MK-2894 sodium salt
Catalog No.:BCC1758
CAS No.:1006036-88-9
- (-)-Epipinoresinol
Catalog No.:BCN3377
CAS No.:10061-38-8
- Desloratadine
Catalog No.:BCC4540
CAS No.:100643-71-8
- Ganoderenic acid A
Catalog No.:BCN3208
CAS No.:100665-40-5
- Ganoderenic acid B
Catalog No.:BCN7966
CAS No.:100665-41-6
- Ganoderenic acid C
Catalog No.:BCN3210
CAS No.:100665-42-7
- Ganoderenic acid D
Catalog No.:BCN2445
CAS No.:100665-43-8
- Deacetyl ganoderic acid F
Catalog No.:BCN2870
CAS No.:100665-44-9
A new diphenyl ether from marine-derived fungus Aspergillus sp. B-F-2.[Pubmed:16915822]
J Antibiot (Tokyo). 2006 Jun;59(6):362-5.
A new diphenyl ether dimethyl 2,3'-dimethylosoate (1) together with three known compounds Monomethylsulochrin (2), emodin (3), and questin (4) were isolated through bioassay-guided fractionations from the culture of a marine-derived fungus Aspergillus sp. B-F-2. The structures of these compounds were determined by spectroscopic methods. Cytotoxicities of compounds 1 and 2 against K562 cell line were preliminarily evaluated by the MTT method and flow cytometry.
Benzophenones from Guignardia sp. IFB-E028, an endophyte on Hopea hainanensis.[Pubmed:20087992]
Chem Biodivers. 2010 Jan;7(1):216-20.
The first natural S-containing benzophenone dimer, named guignasulfide (3), was isolated from the culture of Guignardia sp. IFB-E028, an endophytic fungus residing in healthy leaves of Hopea hainanensis. Its structure was determined through correlative analyses of its MS, 1D- and 2D-NMR spectroscopic data. Two other known benzophenone derivatives, Monomethylsulochrin and rhizoctonic acid (1 and 2, resp.) were also isolated. Guignasulfide (3) was more active against the human liver cancer cell line HepG2 (IC(50) value: 5.2+/-0.4 microM) than metabolites 1 and 2 (IC(50) values: 63.5+/-0.6 and 60.2+/-0.5 microM); compounds 1-3 showed also moderately inhibitory effects on the human bacterial pathogen Helicobacter pylori with MIC values of 28.9+/-0.1, 60.2+/-0.4, and 42.9+/-0.5 microM, respectively.
Chemical constituents of Aspergillus sp EJC08 isolated as endophyte from Bauhinia guianensis and their antimicrobial activity.[Pubmed:24141408]
An Acad Bras Cienc. 2013;85(4):1247-53.
The present work reports the isolation of five compounds from Aspergillus sp EJC08 isolated as endophytic from Bauhinia guianensis, a tipical plant of the Amazon. The compounds ergosterol (1), ergosterol peroxide (2), mevalolactone (3), Monomethylsulochrin (4) and trypacidin A (5) were isolated by chromatographic procedures and identified by spectral methods of 1D and 2D NMR and MS. Compounds 3, 4 and 5 were tested against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Staphylococcus aureus and showed good activity.


