Ganoderenic acid DCAS# 100665-43-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 100665-43-8 | SDF | Download SDF |
| PubChem ID | 91884885 | Appearance | Powder |
| Formula | C30H40O7 | M.Wt | 512.64 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
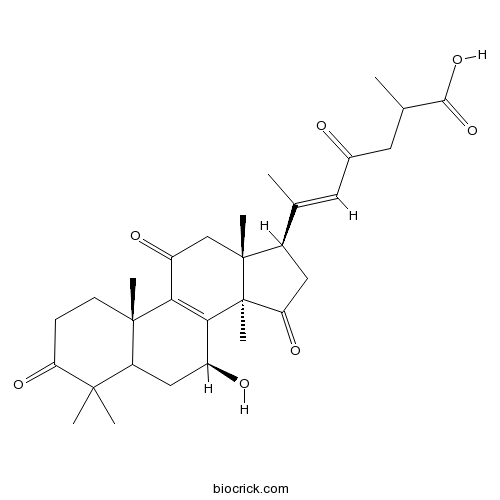
| Chemical Name | (E)-6-[(7S,10S,13R,14R,17R)-7-hydroxy-4,4,10,13,14-pentamethyl-3,11,15-trioxo-1,2,5,6,7,12,16,17-octahydrocyclopenta[a]phenanthren-17-yl]-2-methyl-4-oxohept-5-enoic acid | ||
| SMILES | CC(CC(=O)C=C(C)C1CC(=O)C2(C1(CC(=O)C3=C2C(CC4C3(CCC(=O)C4(C)C)C)O)C)C)C(=O)O | ||
| Standard InChIKey | JGWQYLZHPPFHEH-YZDDURQCSA-N | ||
| Standard InChI | InChI=1S/C30H40O7/c1-15(10-17(31)11-16(2)26(36)37)18-12-23(35)30(7)25-19(32)13-21-27(3,4)22(34)8-9-28(21,5)24(25)20(33)14-29(18,30)6/h10,16,18-19,21,32H,8-9,11-14H2,1-7H3,(H,36,37)/b15-10+/t16?,18-,19+,21?,28+,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ganoderenic acid D is most cytotoxic with IC50 values of 0.14 ± 0.01, 0.18 ± 0.02 and 0.26 ± 0.03 mg/mL in Hep G2, Hela and Caco-2 cells, respectively. |
| Structure Identification | Natural Product Research, Volume 28, Number 24, 17 December 2014, pp. 2264-2272(9)Extraction optimisation and isolation of triterpenoids from Ganoderma lucidum and their effect on human carcinoma cell growth[Reference: WebLink]The response surface methodology was used to optimise the extraction conditions of Ganoderma lucidum based on a Box–Behnken design. |

Ganoderenic acid D Dilution Calculator

Ganoderenic acid D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9507 mL | 9.7534 mL | 19.5069 mL | 39.0137 mL | 48.7672 mL |
| 5 mM | 0.3901 mL | 1.9507 mL | 3.9014 mL | 7.8027 mL | 9.7534 mL |
| 10 mM | 0.1951 mL | 0.9753 mL | 1.9507 mL | 3.9014 mL | 4.8767 mL |
| 50 mM | 0.039 mL | 0.1951 mL | 0.3901 mL | 0.7803 mL | 0.9753 mL |
| 100 mM | 0.0195 mL | 0.0975 mL | 0.1951 mL | 0.3901 mL | 0.4877 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganoderenic acid C
Catalog No.:BCN3210
CAS No.:100665-42-7
- Ganoderenic acid B
Catalog No.:BCN7966
CAS No.:100665-41-6
- Ganoderenic acid A
Catalog No.:BCN3208
CAS No.:100665-40-5
- Desloratadine
Catalog No.:BCC4540
CAS No.:100643-71-8
- (-)-Epipinoresinol
Catalog No.:BCN3377
CAS No.:10061-38-8
- MK-2894 sodium salt
Catalog No.:BCC1758
CAS No.:1006036-88-9
- MK-2894
Catalog No.:BCC1757
CAS No.:1006036-87-8
- NF 546
Catalog No.:BCC7804
CAS No.:1006028-37-0
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- TC ASK 10
Catalog No.:BCC6301
CAS No.:1005775-56-3
- Monomethylsulochrin
Catalog No.:BCN7255
CAS No.:10056-14-1
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- Deacetyl ganoderic acid F
Catalog No.:BCN2870
CAS No.:100665-44-9
- 3-(Hydroxymethyl)cyclopentanol
Catalog No.:BCN5822
CAS No.:1007125-14-5
- CH5132799
Catalog No.:BCC4991
CAS No.:1007207-67-1
- 1-O-Deacetylkhayanolide E
Catalog No.:BCN5823
CAS No.:1007387-95-2
- Ganoderic acid M
Catalog No.:BCN2871
CAS No.:100761-17-9
- (R)-5-Hydroxy-1,7-diphenyl-3-heptanone
Catalog No.:BCN3591
CAS No.:100761-20-4
- Chlorahololide C
Catalog No.:BCN7256
CAS No.:1007859-25-7
- 4,4'-Bis(α,α-dimethylbenzyl)diphenylamine
Catalog No.:BCC8661
CAS No.:10081-67-1
- (RS)-CPP
Catalog No.:BCC6561
CAS No.:100828-16-8
- Piceatannol
Catalog No.:BCN5824
CAS No.:10083-24-6
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
[Ganoderma triterpenoids from aqueous extract of Ganoderma lucidum].[Pubmed:29090550]
Zhongguo Zhong Yao Za Zhi. 2017 May;42(10):1908-1915.
A new triterpenoid and 18 analogues were isolated from the water extract of Ganoderma lucidum by column chromatographic techniques, including silica gel, ODS, Sephadex LH-20, and HPLC. The new compound was elucidated as 2beta-acetoxy-3beta,25-dihydroxy-7,11,15-trioxo-lanost-8-en-26-oic acid on the basis of analyses of extensive spectroscopic data and its physicochemical properties. Comparison of NMR data with those reported in literature, the known analogues were determined as ganoderic acid H (2), 12beta-acetoxy-3beta,7beta-dihydroxy-11,15,23-trioxo-lanost-8,16-dien-26-oic acid (3), Ganoderenic acid D (4),ganoderic acid C1 (5),ganoderic acid G (6),3beta,7beta-dihydroxy-11,15,23-trioxo-lanost-8,16-dien-26-oic acid (7),ganoderic acid B (8),ganoderic acid C6 (9),3beta,15alpha-dihydroxy-7,11,23-trioxo-lanost-8,16-dien-26-oic acid (10),ganoderic acid A (11),ganolucidic acid A (12),lucidenic acid E2 (13),lucidenic acid N (14),lucidenic acid P (15), lucidenic acid B (16),lucidenic acid A (17),lucidenic acid C (18),and lucidenic acid L (19), respectively. Compound 1 is new compound and compounds 2-19 have been reported from G. lucidum. The present study enriches the knowledge of the chemical constituent of G. lucidum and completes chemical investigation of water decoction that is traditional use of G. lucidum.
Network pharmacology analysis of the anti-cancer pharmacological mechanisms of Ganoderma lucidum extract with experimental support using Hepa1-6-bearing C57 BL/6 mice.[Pubmed:28882624]
J Ethnopharmacol. 2018 Jan 10;210:287-295.
ETHNOPHARMACOLOGICAL RELEVANCE: Ganoderma lucidum (GL) is an oriental medical fungus, which was used to prevent and treat many diseases. Previously, the effective compounds of Ganoderma lucidum extract (GLE) were extracted from two kinds of GL, [Ganoderma lucidum (Leyss. Ex Fr.) Karst.] and [Ganoderma sinense Zhao, Xu et Zhang], which have been used for adjuvant anti-cancer clinical therapy for more than 20 years. However, its concrete active compounds and its regulation mechanisms on tumor are unclear. AIM OF THE STUDY: In this study, we aimed to identify the main active compounds from GLE and to investigate its anti-cancer mechanisms via drug-target biological network construction and prediction. MATERIALS AND METHODS: The main active compounds of GLE were identified by HPLC, EI-MS and NMR, and the compounds related targets were predicted using docking program. To investigate the functions of GL holistically, the active compounds of GL and related targets were predicted based on four public databases. Subsequently, the Identified-Compound-Target network and Predicted-Compound-Target network were constructed respectively, and they were overlapped to detect the hub potential targets in both networks. Furthermore, the qRT-PCR and western-blot assays were used to validate the expression levels of target genes in GLE treated Hepa1-6-bearing C57 BL/6 mice. RESULTS: In our work, 12 active compounds of GLE were identified, including Ganoderic acid A, Ganoderenic acid A, Ganoderic acid B, Ganoderic acid H, Ganoderic acid C2, Ganoderenic acid D, Ganoderic acid D, Ganoderenic acid G, Ganoderic acid Y, Kaemferol, Genistein and Ergosterol. Using the docking program, 20 targets were mapped to 12 compounds of GLE. Furthermore, 122 effective active compounds of GL and 116 targets were holistically predicted using public databases. Compare with the Identified-Compound-Target network and Predicted-Compound-Target network, 6 hub targets were screened, including AR, CHRM2, ESR1, NR3C1, NR3C2 and PGR, which was considered as potential markers and might play important roles in the process of GLE treatment. GLE effectively inhibited tumor growth in Hepa1-6-bearing C57 BL/6 mice. Finally, consistent with the results of qRT-PCR data, the results of western-blot assay demonstrated the expression levels of PGR and ESR1 were up-regulated, as well as the expression levels of NR3C2 and AR were down-regulated, while the change of NR3C1 and CHRM2 had no statistical significance. CONCLUSIONS: The results indicated that these 4 hub target genes, including NR3C2, AR, ESR1 and PGR, might act as potential markers to evaluate the curative effect of GLE treatment in tumor. And, the combined data provide preliminary study of the pharmacological mechanisms of GLE, which may be a promising potential therapeutic and chemopreventative candidate for anti-cancer.
Extraction optimisation and isolation of triterpenoids from Ganoderma lucidum and their effect on human carcinoma cell growth.[Pubmed:25032738]
Nat Prod Res. 2014;28(24):2264-72.
The response surface methodology was used to optimise the extraction conditions of Ganoderma lucidum based on a Box-Behnken design. A quadratic model sufficiently simulated the response of ganoderic acid H with a determination coefficient (R(2)) of 0.98. The optimal condition for extracting triterpenoids was determined to be 100.00% ethanol at 60.22 degrees C for 6.00 h, under which the yield of the reference triterpenoid ganoderic acid H increased from 0.88 to 2.09 mg/g powder. Following extraction, triterpenoid-enriched fraction was further isolated into 23 fractions, and 7 fractions were identified as ganoderic acids A, B, D, G, H and I and Ganoderenic acid D. Of the seven triterpenoids, Ganoderenic acid D was most cytotoxic with IC50 values of 0.14 +/- 0.01, 0.18 +/- 0.02 and 0.26 +/- 0.03 mg/mL in Hep G2, Hela and Caco-2 cells, respectively. While ganoderic acids A, G and H were relatively non-cytotoxic. The variation of inhibitory effects for these triterpenoids was likely related to their chemical structures.
[Determination of nine triterpenoid acids from Ganoderma lucidum of different producting areas by HPLC].[Pubmed:23477148]
Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3599-603.
OBJECTIVE: To establish an HPLC method for determining nine triterpenes contained in Ganoderma lucidum. METHOD: Chromatography conditions: Alltima C18 (4.6 mm x 150 mm, 5 microm) was adopted as the chromatographic column, with acetonitrile-0.04% formic acid solution as the mobile phase. The detective wavelength was set at 254 nm, and the column temperature was 15 degrees C. RESULT: The linearities of ganoderic acid C2, ganoderic acid G, ganoderenic acid B, ganoderic acid B, ganoderenic acid A, ganoderic acid A, lucideric acid A, Ganoderenic acid D, and ganoderic acid C1 ranged between 6.81-40.88, 6.38-38.25, 6.75-40.50, 6.38-38.25, 5.95-35.65, 5.90-35.25, 7.00-42.00, 6.20-37.15 and 6.05-36.4 mg x L(-1) (r = 0.999 4, 0.999 2, 0.999 4, 0.999 2, 0.999 2, 0.994 5, 0.999 0, 0.999 2 and 0.998 4). Their recoveries were 102.1%, 102.3%, 100.6%, 103.3%, 104.1%, 103.2%, 96.42%, 102.5% and 101.5%, with RSD being 1.5%, 0.96%, 1.9%, 1.3%, 1.7%, 2.5%, 0.62%, 2.9% and 1.3%. The content of triterpenes contained in G. lucidum samples from 31 different areas and under different cultivation conditions. CONCLUSION: The method is so feasible and highly reproducible that it can be used for quantitatie determination of the content of triterpenoid acid contained in G. lucidum.
Constituents of various wood-rotting basidiomycetes.[Pubmed:10963454]
Phytochemistry. 2000 Jul;54(6):603-10.
Phytochemical investigation of n-hexane and methanol extracts of fruiting bodies of the wood-rotting fungi Fomitopsis pinicola. Ganoderma lipsiense, Fomes fomentarius and Gloeophyllum odoratum led to the isolation and identification of several triterpene derivatives and some aromatic compounds derived from lignin. These are the new natural products, namely, pinicolic acid E (16alpha-hydroxy-3-oxolanosta-8,24-dien-21-oic acid) and pinicolol C (3-oxolanosta-7,9(11),24-trien-15alpha,21-diol) from the crust of F. pinicola, Ganoderenic acid D [(E)-7beta-hydroxy-3,11,15,23-tetraoxolanosta-8,20(22)-di en-26-oic acid] and ganoderic acid N (7beta,20-dihydroxy-3,11,15,23-tetraoxolanost-8-en-26-oic acid) from G. lipsiense and ergosterol peroxide (5alpha,8alpha-epi-dioxyergost-6-en-3beta-ol) as well as ergost-7-en-3-one from F. fomentarius. From G. odoratum, dehydroeburicoic acid [24-methylene-3-oxolanosta-7,9(11)-dien-21-oic acid], the dimethylacetal of 4,4,14alpha-trimethyl-24-oxo-5alpha-chol-8-en-21-oic acid and some aromatic compounds, of which 1-(4'-methoxyphenyl)-1,2-ethandiol is a new natural product, were isolated. Furthermore, a complete set of 13C NMR data of the steryl esters 3beta-linoleyloxyergosta-7,24(28)-diene, 3beta-linoleyloxyergosta-7,24-diene and 3beta-linoleyloxyergost-7-ene, which could be identified as a mixture in all investigated fungi, could be recorded. It was proved by HPLC and TLC investigations, that the crust on top of the fruiting bodies of F. pinicola consists of lanostane derivatives.


