LupanineCAS# 550-90-3 |
- alpha-Isolupanine
Catalog No.:BCN7989
CAS No.:486-87-3
Quality Control & MSDS
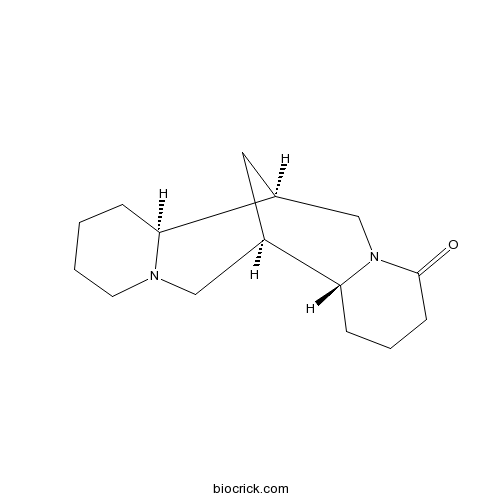
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 550-90-3 | SDF | Download SDF |
| PubChem ID | 442956 | Appearance | Brown oil |
| Formula | C15H24N2O | M.Wt | 248.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | 2-Oxosparteine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | C1CCN2CC3CC(C2C1)CN4C3CCCC4=O | ||
| Standard InChIKey | JYIJIIVLEOETIQ-ZOBORPQBSA-N | ||
| Standard InChI | InChI=1S/C15H24N2O/c18-15-6-3-5-14-11-8-12(10-17(14)15)13-4-1-2-7-16(13)9-11/h11-14H,1-10H2/t11-,12+,13+,14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Lupanine improves glucose homeostasis by influencing KATP-channels of pancreatic beta cells. 2. Lupanine has a weak sedative effect on the central nervous system, interaction with specific drugs used for treatment of the CNS and for analgesic activity. |
| Targets | Calcium Channel | ATPase | Potassium Channel |

Lupanine Dilution Calculator

Lupanine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0258 mL | 20.1288 mL | 40.2576 mL | 80.5153 mL | 100.6441 mL |
| 5 mM | 0.8052 mL | 4.0258 mL | 8.0515 mL | 16.1031 mL | 20.1288 mL |
| 10 mM | 0.4026 mL | 2.0129 mL | 4.0258 mL | 8.0515 mL | 10.0644 mL |
| 50 mM | 0.0805 mL | 0.4026 mL | 0.8052 mL | 1.6103 mL | 2.0129 mL |
| 100 mM | 0.0403 mL | 0.2013 mL | 0.4026 mL | 0.8052 mL | 1.0064 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Afrormosine
Catalog No.:BCN3312
CAS No.:550-79-8
- trans-Triprolidine hydrochloride
Catalog No.:BCC6742
CAS No.:550-70-9
- Angustifoline
Catalog No.:BCN3205
CAS No.:550-43-6
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- Busulfan
Catalog No.:BCC3742
CAS No.:55-98-1
- Hexamethonium Bromide
Catalog No.:BCC4561
CAS No.:55-97-0
- 1-Phenylbiguanide hydrochloride
Catalog No.:BCC6870
CAS No.:55-57-2
- Atropine sulfate
Catalog No.:BCN2716
CAS No.:55-48-1
- McN-A 343
Catalog No.:BCC7042
CAS No.:55-45-8
- Epinephrine HCl
Catalog No.:BCC4319
CAS No.:55-31-2
- Benzamide
Catalog No.:BCN5737
CAS No.:55-21-0
- HYOSCINE HYDROCHLORIDE
Catalog No.:BCN8331
CAS No.:55-16-3
- Naphazoline HCl
Catalog No.:BCC4331
CAS No.:550-99-2
- NAADP tetrasodium salt
Catalog No.:BCC7808
CAS No.:5502-96-5
- (±)-Cloprostenol sodium salt
Catalog No.:BCC7315
CAS No.:55028-72-3
- Isorhamnetin-3-O-neohespeidoside
Catalog No.:BCN1234
CAS No.:55033-90-4
- Dammar-20(21)-en-3,24,25-triol
Catalog No.:BCN5734
CAS No.:55050-69-6
- 2,6,4'-Trihydroxy-4-methoxybenzophenone
Catalog No.:BCN7588
CAS No.:55051-85-9
- Protodioscin
Catalog No.:BCN6274
CAS No.:55056-80-9
- Diversoside
Catalog No.:BCN7537
CAS No.:55062-36-7
- Acitretin
Catalog No.:BCC1189
CAS No.:55079-83-9
- Andrographolide
Catalog No.:BCN5735
CAS No.:5508-58-7
- Skullcapflavone II
Catalog No.:BCN3188
CAS No.:55084-08-7
- 5,6,7-Trimethoxycoumarin
Catalog No.:BCN7590
CAS No.:55085-47-7
Uptake of Lupanine by Alkaloid-Storing Epidermal Cells of Lupinus polyphyllus.[Pubmed:17269069]
Planta Med. 1987 Oct;53(5):465-9.
Epidermis of steins and petioles of LUPINUS POLYPHYLLUS accumulates quinolizidine alkaloids at a concentration of about 30 mM. Since Lupanine is synthesized mainly in green mesophyll tissue and not in the epidermis, the alkaloids have to be transported into the epidermal cells. Uptake of [ (3)H]-Lupanine into isolated epidermis was 3 to 20 times higher in epidermal cells as compared to the corresponding mesophyll cells. Uptake of Lupanine is time dependent and proceeds against a concentration gradient. The uptake depends on temperature and can be characterized by an activation energy of 34 kJ/mol. The process shows multiphasic uptake kinetics and is reduced by SH-group inhibitors (NEM, PHMB) and inhibitors of the energy metabolism (cyanide, antimycine, DNP, CCCP). All these data provide first evidence that simple diffusion cannot be the mechanism for the uptake of Lupanine into epidermal cells. The uptake is probably catalyzed by transport proteins.
A comparative study of the effects of sparteine, lupanine and lupin extract on the central nervous system of the mouse.[Pubmed:9751462]
J Pharm Pharmacol. 1998 Aug;50(8):949-54.
Lupin is toxic because of its alkaloid content, sparteine and Lupanine in particular. Although the pharmacological properties of sparteine are well known those of Lupanine have not been much studied. This paper reports procedures for extraction, purification and crystallization of Lupanine, and methods for the preparation of an extract for injection of Lupinus mutabilis Sweet, and for the determination of the acute toxicity and maximum non-lethal dose (DL0) of Lupanine, sparteine and lupin extract in the mouse. The three substances were tested on the central nervous system (CNS) for locomotor activity, for interaction with specific drugs used for treatment of the CNS (the stimulant drugs amphetamine and pentetrazol and the depressant drugs pentobarbital and chlorpromazine) and for analgesic activity. The results indicate that Lupanine and lupin extract are less toxic than sparteine and that at the doses studied the three products have a weak sedative effect on the CNS.
Lupanine Improves Glucose Homeostasis by Influencing KATP Channels and Insulin Gene Expression.[Pubmed:26492234]
Molecules. 2015 Oct 20;20(10):19085-100.
The glucose-lowering effects of lupin seeds involve the combined action of several components. The present study investigates the influence of one of the main quinolizidine alkaloids, Lupanine, on pancreatic beta cells and in an animal model of type-2 diabetes mellitus. In vitro studies were performed with insulin-secreting INS-1E cells or islets of C57BL/6 mice. In the in vivo experiments, hyperglycemia was induced in rats by injecting streptozotocin (65 mg/kg body weight). In the presence of 15 mmol/L glucose, insulin secretion was significantly elevated by 0.5 mmol/L Lupanine, whereas the alkaloid did not stimulate insulin release with lower glucose concentrations. In islets treated with l-arginine, the potentiating effect of Lupanine already occurred at 8 mmol/L glucose. Lupanine increased the expression of the Ins-1 gene. The potentiating effect on secretion was correlated to membrane depolarization and an increase in the frequency of Ca(2+) action potentials. Determination of the current through ATP-dependent K(+) channels (KATP channels) revealed that Lupanine directly inhibited the channel. The effect was dose-dependent but, even with a high Lupanine concentration of 1 mmol/L or after a prolonged exposure time (12 h), the KATP channel block was incomplete. Oral administration of Lupanine did not induce hypoglycemia. By contrast, Lupanine improved glycemic control in response to an oral glucose tolerance test in streptozotocin-diabetic rats. In summary, Lupanine acts as a positive modulator of insulin release obviously without a risk for hypoglycemic episodes.


