5,6,7-TrimethoxycoumarinCAS# 55085-47-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55085-47-7 | SDF | Download SDF |
| PubChem ID | 148724 | Appearance | Powder |
| Formula | C12H12O5 | M.Wt | 236.22 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
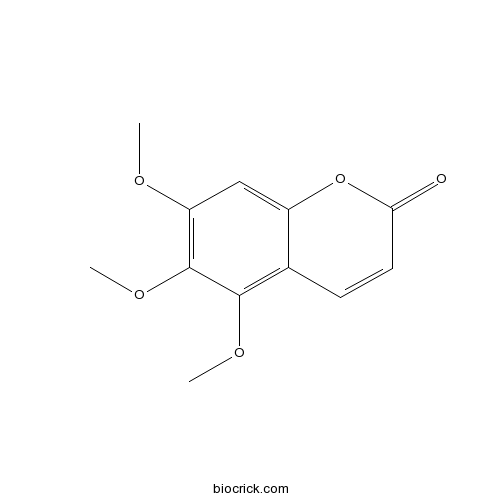
| Chemical Name | 5,6,7-trimethoxychromen-2-one | ||
| SMILES | COC1=C(C(=C2C=CC(=O)OC2=C1)OC)OC | ||
| Standard InChIKey | FOBNRKTURPWTQX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H12O5/c1-14-9-6-8-7(4-5-10(13)17-8)11(15-2)12(9)16-3/h4-6H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 5,6,7-Trimethoxycoumarin can improve gastroprotective effects, and it has low toxicity and minimal effects on drug-metabolizing enzymes. 2. 5,6,7-Trimethoxycoumarin shows moderate inhibitory activity against Micrococcus luteus. 3. 5,6,7-Trimethoxycoumarin displays an intermediate cytotoxic effects against brine shrimp larvae. |
| Targets | P450 (e.g. CYP17) | Antifection |

5,6,7-Trimethoxycoumarin Dilution Calculator

5,6,7-Trimethoxycoumarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2333 mL | 21.1667 mL | 42.3334 mL | 84.6668 mL | 105.8335 mL |
| 5 mM | 0.8467 mL | 4.2333 mL | 8.4667 mL | 16.9334 mL | 21.1667 mL |
| 10 mM | 0.4233 mL | 2.1167 mL | 4.2333 mL | 8.4667 mL | 10.5834 mL |
| 50 mM | 0.0847 mL | 0.4233 mL | 0.8467 mL | 1.6933 mL | 2.1167 mL |
| 100 mM | 0.0423 mL | 0.2117 mL | 0.4233 mL | 0.8467 mL | 1.0583 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Skullcapflavone II
Catalog No.:BCN3188
CAS No.:55084-08-7
- Andrographolide
Catalog No.:BCN5735
CAS No.:5508-58-7
- Acitretin
Catalog No.:BCC1189
CAS No.:55079-83-9
- Diversoside
Catalog No.:BCN7537
CAS No.:55062-36-7
- Protodioscin
Catalog No.:BCN6274
CAS No.:55056-80-9
- 2,6,4'-Trihydroxy-4-methoxybenzophenone
Catalog No.:BCN7588
CAS No.:55051-85-9
- Dammar-20(21)-en-3,24,25-triol
Catalog No.:BCN5734
CAS No.:55050-69-6
- Isorhamnetin-3-O-neohespeidoside
Catalog No.:BCN1234
CAS No.:55033-90-4
- (±)-Cloprostenol sodium salt
Catalog No.:BCC7315
CAS No.:55028-72-3
- NAADP tetrasodium salt
Catalog No.:BCC7808
CAS No.:5502-96-5
- Naphazoline HCl
Catalog No.:BCC4331
CAS No.:550-99-2
- Lupanine
Catalog No.:BCN5736
CAS No.:550-90-3
- Nalmefene - d3
Catalog No.:BCC6093
CAS No.:55096-26-9
- EVP-6124 hydrochloride
Catalog No.:BCC1567
CAS No.:550999-74-1
- EVP-6124
Catalog No.:BCC1566
CAS No.:550999-75-2
- 3-Butylidenephthalide
Catalog No.:BCN6345
CAS No.:551-08-6
- Liquiritin
Catalog No.:BCN5944
CAS No.:551-15-5
- 6-Aminopenicillanic acid
Catalog No.:BCC8765
CAS No.:551-16-6
- Viridiflorine
Catalog No.:BCN2045
CAS No.:551-57-5
- Supinine
Catalog No.:BCN1952
CAS No.:551-58-6
- Dimetridazole
Catalog No.:BCC8944
CAS No.:551-92-8
- 2'-Aminoacetophenone
Catalog No.:BCN1746
CAS No.:551-93-9
- 4beta-Carboxy-19-nortotarol
Catalog No.:BCN4065
CAS No.:55102-39-1
- (S)-3-Carboxy-4-hydroxyphenylglycine
Catalog No.:BCC6607
CAS No.:55136-48-6
Gastroprotective efficacy and safety evaluation of scoparone derivatives on experimentally induced gastric lesions in rodents.[Pubmed:25781220]
Nutrients. 2015 Mar 13;7(3):1945-64.
This study investigated the gastroprotective efficacy of synthesized scoparone derivatives on experimentally induced gastritis and their toxicological safety. Six scoparone derivatives were synthesized and screened for gastroprotective activities against HCl/ethanol- and indomethacin-induced gastric ulcers in rats. Among these compounds, 5,6,7-Trimethoxycoumarin and 6,7,8-trimethoxycoumarin were found to have gastroprotective activity greater than the standard drug rebamipide; 6-methoxy-7,8-methylenedioxycoumarin, 6-methoxy-7,8-(1-methoxy)-methylenedioxycoumarin, 6,7-methylenedioxycoumarin, and 6,7-(1-methoxy)-methylenedioxycoumarin were found to be equipotent or less potent that of rebamipide. Pharmacological studies suggest that the presence of a methoxy group at position C-5 or C-8 of the scoparone's phenyl ring significantly improves gastroprotective activity, whereas the presence of a dioxolane ring at C-6, C-7, or C-8 was found to have decreased activity. In order to assess toxicological safety, two of the potent gastroprotective scoparone derivatives-5,6,7-Trimethoxycoumarin and 6,7,8-trimethoxycoumarin-were examined for their acute toxicity in mice as well as their effect on cytochrome P450 (CYP) enzyme activity. These two compounds showed low acute oral toxicity in adult male and female mice, and caused minimal changes to CYP3A4 and CYP2C9 enzyme activity. These results indicate that compared to other scoparone derivatives, 5,6,7-Trimethoxycoumarin and 6,7,8-trimethoxycoumarin can improve gastroprotective effects, and they have low toxicity and minimal effects on drug-metabolizing enzymes.
Irritant and cytotoxic coumarins from Angelica glauca Edgew roots.[Pubmed:18058380]
J Asian Nat Prod Res. 2008 Jan-Feb;10(1-2):49-58.
Irritant and cytotoxic potentiality of six coumarins, isolated for the first time from the roots of Angelica glauca identified as 5,6,7-Trimethoxycoumarin, 6-methoxy-7,8-methylenedioxycoumarin, bergapten, decursinol angelate, decursin, and nodakenetin, were investigated. The irritant potential was explored by open mouse ear assay, evaluating their ID(50) after acute and by IU (Irritant units) after chronic effects, while the cytotoxic capability was explored by their LC(50), using brine shrimp (Artemia salina) larvae (nauplii). All the coumarins exhibited well-defined irritancy on mouse's ears, compared with the positive controlled euphorbium reaction and cytotoxic response against brine shrimp larvae, compared with the positive control colchicine. Decursinol angelate and decursin were the most potent and persistent irritant compounds with least ID(50), whose reactions lasted for 48 h. 6-Methoxy-7,8-methylenedioxycoumarin and bergaten revealed an intermediate irritant reactions, while 5,6,7-Trimethoxycoumarin and nodakenetin displayed the least irritant and least persistent reactions on mouse ears. Both decursin and decursinol angelate also appeared to be the stronger cytotoxic agents than other coumarins. 5,6,7-Trimethoxycoumarin displayed an intermediate cytotoxic behaviour, while other three coumarins, i.e., 6-methoxy-7,8-methylenedioxycoumarin, bergapten, and nodakenetin, exhibited the least cytotoxic capacity against brine shrimp larvae.
Two new coumarin glycosides from Chimonanthus nitens.[Pubmed:23421779]
J Asian Nat Prod Res. 2013;15(3):270-5.
Two new coumarin glycosides, namely nitensosides A-B (1-2), together with six known compounds, scopolin (3), 5,6,7-Trimethoxycoumarin (4), d-calycanthine (5), calycanthoside (6), xeroboside (7), and scopoletin (8), were isolated from Chimonanthus nitens. The structures of the new compounds were elucidated by comprehensive analysis of IR, MS, and NMR spectroscopic data. Compounds 3, 4, 7, and 8 showed moderate inhibitory activity against Micrococcus luteus.


