2'-AminoacetophenoneCAS# 551-93-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 551-93-9 | SDF | Download SDF |
| PubChem ID | 11086 | Appearance | Powder |
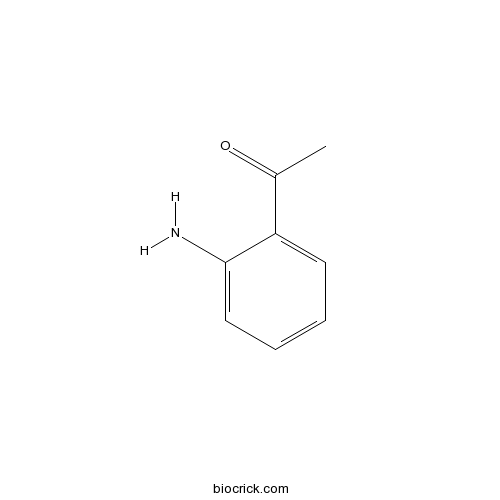
| Formula | C8H9NO | M.Wt | 135.16 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(2-aminophenyl)ethanone | ||
| SMILES | CC(=O)C1=CC=CC=C1N | ||
| Standard InChIKey | GTDQGKWDWVUKTI-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. A new route to oxcarbazepine (Trileptal), the most widely prescribed antiepileptic drug, starting from commercially available 2'-aminoacetophenone and 1,2-dibromobenzene, is reported. |

2'-Aminoacetophenone Dilution Calculator

2'-Aminoacetophenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3986 mL | 36.9932 mL | 73.9864 mL | 147.9728 mL | 184.966 mL |
| 5 mM | 1.4797 mL | 7.3986 mL | 14.7973 mL | 29.5946 mL | 36.9932 mL |
| 10 mM | 0.7399 mL | 3.6993 mL | 7.3986 mL | 14.7973 mL | 18.4966 mL |
| 50 mM | 0.148 mL | 0.7399 mL | 1.4797 mL | 2.9595 mL | 3.6993 mL |
| 100 mM | 0.074 mL | 0.3699 mL | 0.7399 mL | 1.4797 mL | 1.8497 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dimetridazole
Catalog No.:BCC8944
CAS No.:551-92-8
- Supinine
Catalog No.:BCN1952
CAS No.:551-58-6
- Viridiflorine
Catalog No.:BCN2045
CAS No.:551-57-5
- 6-Aminopenicillanic acid
Catalog No.:BCC8765
CAS No.:551-16-6
- Liquiritin
Catalog No.:BCN5944
CAS No.:551-15-5
- 3-Butylidenephthalide
Catalog No.:BCN6345
CAS No.:551-08-6
- EVP-6124
Catalog No.:BCC1566
CAS No.:550999-75-2
- EVP-6124 hydrochloride
Catalog No.:BCC1567
CAS No.:550999-74-1
- Nalmefene - d3
Catalog No.:BCC6093
CAS No.:55096-26-9
- 5,6,7-Trimethoxycoumarin
Catalog No.:BCN7590
CAS No.:55085-47-7
- Skullcapflavone II
Catalog No.:BCN3188
CAS No.:55084-08-7
- Andrographolide
Catalog No.:BCN5735
CAS No.:5508-58-7
- 4beta-Carboxy-19-nortotarol
Catalog No.:BCN4065
CAS No.:55102-39-1
- (S)-3-Carboxy-4-hydroxyphenylglycine
Catalog No.:BCC6607
CAS No.:55136-48-6
- Kaempferol 3-sophoroside-7-glucoside
Catalog No.:BCN7825
CAS No.:55136-76-0
- Oxohydrastinine
Catalog No.:BCN3299
CAS No.:552-29-4
- Paeonol
Catalog No.:BCN5738
CAS No.:552-41-0
- Isorhoifolin
Catalog No.:BCN5739
CAS No.:552-57-8
- Eriodictyol
Catalog No.:BCN1209
CAS No.:552-58-9
- Prunetin
Catalog No.:BCN2335
CAS No.:552-59-0
- Daidzin
Catalog No.:BCN5891
CAS No.:552-66-9
- Sasapyrine
Catalog No.:BCC4714
CAS No.:552-94-3
- Dexamethasone Sodium Phosphate
Catalog No.:BCC4557
CAS No.:55203-24-2
- AP 18
Catalog No.:BCC7634
CAS No.:55224-94-7
An advantageous route to oxcarbazepine (trileptal) based on palladium-catalyzed arylations free of transmetallating agents.[Pubmed:16235889]
Org Lett. 2005 Oct 27;7(22):4787-9.
[reaction: see text] A new route to oxcarbazepine (Trileptal), the most widely prescribed antiepileptic drug, starting from commercially available 2'-aminoacetophenone and 1,2-dibromobenzene, is reported. The sequentially accomplished key steps are palladium-catalyzed intermolecular alpha-arylation of ketone enolates and intramolecular N-arylation reactions. After several experiments to establish the best conditions for both arylation processes, the target oxcarbazepine is obtained in a satisfactory overall yield, minimizing the number of steps and employing scalable catalytic procedures developed in partially aqueous media.
Intramolecular and intermolecular hydrogen-bonding effects on photophysical properties of 2'-aminoacetophenone and its derivatives in solution.[Pubmed:15803207]
Photochem Photobiol Sci. 2005 Apr;4(4):367-75.
Effects of intra- and intermolecular hydrogen-bonds on the photophysical properties of 2'-aminoacetophenone derivatives (X-C6H4-COCH3) having a substituted amino group (X) with different hydrogen-bonding ability to the carbonyl oxygen (X: NH2(AAP), NHCH3(MAAP), N(CH3)2(DMAAP), NHCOCH3(AAAP), NHCOCF3(TFAAP)) are investigated by means of steady-state and time-resolved fluorescence spectroscopy and time-resolved thermal lensing. Based on the photophysical parameters obtained in aprotic solvents with different polarity and protic solvents with different hydrogen-bonding ability, the characteristic photophysical behavior of the 2'-aminoacetophenone derivatives is discussed in terms of hydrogen-bonding and n,pi*-pi,pi* vibronic coupling. The dominant deactivation process of AAP and MAAP in nonpolar aprotic solvents is the extremely fast internal conversion (k(ic)= 1.0 x 10(11) s(-1) for AAP and 3.9 x 10(10) s(-1) for MAAP in n-hexane). The internal conversion rates of both compounds decrease markedly with increasing solvent polarity, suggesting that vibronic interactions between close-lying S1(pi,pi*) and S2(n,pi*) states lead to the large increase in the non-radiative decay rate of the lowest excited singlet state. It is also suggested that for MAAP, which has a stronger hydrogen-bond as compared to AAP, an intramolecular hydrogen-bonding induced deactivation is involved in the dissipation of the S1 state. For DMAAP, which cannot possess an intramolecular hydrogen-bond, the primary relaxation mechanism of the S1 state in nonpolar aprotic solvents is the intersystem crossing to the triplet state, whereas in protic solvents very efficient internal conversion due to intermolecular hydrogen-bonding is induced. In contrast, the fluorescence spectra of AAAP and TFAAP, which have an amino group with a much stronger hydrogen-bonding ability, give strongly Stokes-shifted fluorescence, indicating that these compounds undergo excited-state intramolecular proton transfer reaction upon electronic excitation.
Glycosylation of aromatic amines III: Mechanistic implications of the pH-dependent glycosylation of various aromatic amines (kynurenine, 2'-aminoacetophenone, daptomycin, and sulfamethoxzaole).[Pubmed:19551894]
J Pharm Sci. 2009 Dec;98(12):4639-49.
Glycosylation reaction kinetics of a series of aromatic amines (kynurenine, 2'-aminoacetophenone, daptomycin, and sulfamethoxazole) was compared to propose a unifying reaction mechanism. Kinetic studies were conducted in aqueous solutions containing glucose in the pH range 1-6.5 with 2'-aminoacetophenone and daptomycin. The resultant pH-rate profiles were compared to previously reported profiles for the reactions of glucose and kynurenine or sulfamethoxazole. Glycosylation of weakly basic aromatic amines involved the addition of the unprotonated amine to the aldehydic sugar leading to carbinolamine formation followed by specific acid catalyzed dehydration. All of the pH-rate profiles displayed characteristic downward bend at pH 4-5 due to a change from rate-determining addition to dehydration. In the pH-rate profile for kynurenine, a second downward bend was observed in the pH region 2-4. This feature was absent for the other substrates and was attributed to differences in reactivity of the two ionization states of the alpha carboxylic acid in kynurenine. This stabilization was not possible for the other amines studied.


