2,6,4'-Trihydroxy-4-methoxybenzophenoneCAS# 55051-85-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55051-85-9 | SDF | Download SDF |
| PubChem ID | 10467773 | Appearance | Powder |
| Formula | C14H12O5 | M.Wt | 260.24 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
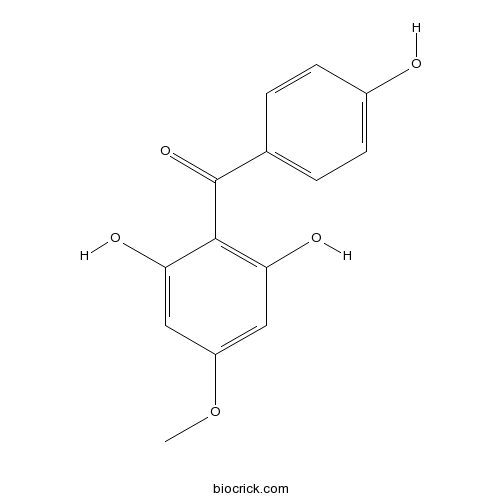
| Chemical Name | (2,6-dihydroxy-4-methoxyphenyl)-(4-hydroxyphenyl)methanone | ||
| SMILES | COC1=CC(=C(C(=C1)O)C(=O)C2=CC=C(C=C2)O)O | ||
| Standard InChIKey | MYEMIGSUACCKND-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H12O5/c1-19-10-6-11(16)13(12(17)7-10)14(18)8-2-4-9(15)5-3-8/h2-7,15-17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 2,6,4'-Trihydroxy-4-methoxybenzophenone has neurotrophic activity, it induced neurite outgrowth in PC-12 cells at concentration of 50 microg/ml. 2. 2,6,4'-Trihydroxy-4-methoxybenzophenone shows weak inhibitory activity of testosterone 5alpha-reductase . 3. 2,6,4′-Trihydroxy-4-methoxybenzophenone exhibits low cytotoxic effect against HeLa and 3T3 cell lines with IC50 values of 132 ug/ml and 158 ug/ml, repectively. 4. 2,6,4′-Trihydroxy-4-methoxybenzophenone shows significant inhibition of pancreatic lipase activity. 5. 2,6,4'-Trihydroxy-4-methoxybenzophenon shows antioxidant activity on DPPH with IC50 of 10.57 ug/mL. |
| Targets | Bcl-2/Bax |

2,6,4'-Trihydroxy-4-methoxybenzophenone Dilution Calculator

2,6,4'-Trihydroxy-4-methoxybenzophenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8426 mL | 19.213 mL | 38.4261 mL | 76.8521 mL | 96.0652 mL |
| 5 mM | 0.7685 mL | 3.8426 mL | 7.6852 mL | 15.3704 mL | 19.213 mL |
| 10 mM | 0.3843 mL | 1.9213 mL | 3.8426 mL | 7.6852 mL | 9.6065 mL |
| 50 mM | 0.0769 mL | 0.3843 mL | 0.7685 mL | 1.537 mL | 1.9213 mL |
| 100 mM | 0.0384 mL | 0.1921 mL | 0.3843 mL | 0.7685 mL | 0.9607 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dammar-20(21)-en-3,24,25-triol
Catalog No.:BCN5734
CAS No.:55050-69-6
- Isorhamnetin-3-O-neohespeidoside
Catalog No.:BCN1234
CAS No.:55033-90-4
- (±)-Cloprostenol sodium salt
Catalog No.:BCC7315
CAS No.:55028-72-3
- NAADP tetrasodium salt
Catalog No.:BCC7808
CAS No.:5502-96-5
- Naphazoline HCl
Catalog No.:BCC4331
CAS No.:550-99-2
- Lupanine
Catalog No.:BCN5736
CAS No.:550-90-3
- Afrormosine
Catalog No.:BCN3312
CAS No.:550-79-8
- trans-Triprolidine hydrochloride
Catalog No.:BCC6742
CAS No.:550-70-9
- Angustifoline
Catalog No.:BCN3205
CAS No.:550-43-6
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- Busulfan
Catalog No.:BCC3742
CAS No.:55-98-1
- Hexamethonium Bromide
Catalog No.:BCC4561
CAS No.:55-97-0
- Protodioscin
Catalog No.:BCN6274
CAS No.:55056-80-9
- Diversoside
Catalog No.:BCN7537
CAS No.:55062-36-7
- Acitretin
Catalog No.:BCC1189
CAS No.:55079-83-9
- Andrographolide
Catalog No.:BCN5735
CAS No.:5508-58-7
- Skullcapflavone II
Catalog No.:BCN3188
CAS No.:55084-08-7
- 5,6,7-Trimethoxycoumarin
Catalog No.:BCN7590
CAS No.:55085-47-7
- Nalmefene - d3
Catalog No.:BCC6093
CAS No.:55096-26-9
- EVP-6124 hydrochloride
Catalog No.:BCC1567
CAS No.:550999-74-1
- EVP-6124
Catalog No.:BCC1566
CAS No.:550999-75-2
- 3-Butylidenephthalide
Catalog No.:BCN6345
CAS No.:551-08-6
- Liquiritin
Catalog No.:BCN5944
CAS No.:551-15-5
- 6-Aminopenicillanic acid
Catalog No.:BCC8765
CAS No.:551-16-6
7-hydroxy-3-(4-hydroxybenzyl)chroman and broussonin b: neurotrophic compounds, isolated from Anemarrhena asphodeloides BUNGE, function as proteasome inhibitors.[Pubmed:16141565]
Biol Pharm Bull. 2005 Sep;28(9):1798-800.
The extract of Anemarrhenae Rhizoma (rhizomes of Anemarrhena asphodeloides BUNGE) showed neurotrophic activity toward rat pheochromocytoma (PC-12) cells. Bioassay-guided purification afforded four compounds, 2,6,4'-trihydroxy-4-methoxybenzophenone (1), 7-hydroxy-3-(4-hydroxybenzyl)chroman (2), broussonin B (3), and cis-hinokiresinol (4). Compounds 1-3 induced neurite outgrowth in PC-12 cells at concentration of 50 microg/ml, while 4 was less active. In addition, compounds 2-4 showed moderate inhibitory activities against a chymotrypsin-like activity of the proteasome.
Induction of apoptosis of 2,4',6-trihydroxybenzophenone in HT-29 colon carcinoma cell line.[Pubmed:24579081]
Biomed Res Int. 2014;2014:468157.
2,4',6-Trihydroxy-4-methoxybenzophenone was isolated from the ethyl acetate fraction of Phaleria macrocarpa (Scheff.) Boerl. fruits. It was found to inhibit cell proliferation in HT-29 human colon carcinoma cell line but caused little damage to WRL-68 normal human liver and MRC-5 normal human fibroblast lung cell lines. The compound was found to sharply affect the viability of HT-29 cells in a dose- and time-dependent manner. HT-29 cells treated with the compound showed morphological changes under microscopic examination such as cell shrinkage, membrane blebbing, DNA fragmentation, and the occurrence of apoptotic nuclei. The percentage of early apoptotic, late apoptotic, and dead or necrotic cells was determined by flow cytometry using annexin V-FTIC/PI staining. In addition, flow cytometry showed that, when the HT-29 cells were treated with 115 microM of the compound, it resulted in G0/G1 phase arrest in a time-dependent manner. Western blot revealed an upregulation of PUMA, Bak, Bcl-2, and Mcl-1 proteins suggesting that the compound induced apoptosis in HT-29 cells by regulating these proteins.
Testosterone 5alpha-reductase inhibitory active constituents from Anemarrhenae Rhizoma.[Pubmed:11379787]
Biol Pharm Bull. 2001 May;24(5):586-7.
The diethyl ether extract of Anemarrhenae Rhizoma (rhizomes of Anemarrhena asphodeloides Bunge) showed testosterone 5alpha-reductase inhibitory activity. Two major constituents, cis-hinokiresinol (1) and 2,6,4'-trihydroxy-4-methoxybenzophenone (2) were identified as the active principles. The inhibitory activity of 1 was superior to that of ethinylestradiol, but that of 2 was weak.
Inhibitory activity of benzophenones from Anemarrhena asphodeloides on pancreatic lipase.[Pubmed:23738459]
Nat Prod Commun. 2013 Apr;8(4):481-3.
Pancreatic lipase is a key enzyme for lipid absorption by hydrolysis of total dietary fats. Therefore, inhibition of pancreatic lipase is suggested to be an effective therapy in the regulation of obesity. The EtOAc-soluble fraction of Anemarrhena asphodeloides rhizomes significantly inhibited pancreatic lipase activity as assessed using porcine pancreatic lipase as an in vitro assay system. Further fractionation of the EtOAc-soluble fraction of A. asphodeloides led to the isolation of a new benzophenone glycoside, zimoside A (1), together with the eleven known compounds iriflophenone (2), 2,4',6-trihydroxy-4-methoxybenzophenone (3), foliamangiferoside A (4), (2,3-dihydroxy-4-methoxyphenyl)(4-hydroxyphenyl)-methanone (5), 1,4,5,6,-tetrahydroxyxanthone (6), isosakuranetin (7), 4-hydroxybenzoic acid (8), 4-hydroxyacetophenone (9), vanillic acid (10), tyrosol (11) and 5-hydroxymethyl-2-furaldehyde (12). Among the isolated compounds, 3, 5 and 10 showed significant inhibition of pancreatic lipase activity.


