AngustifolineCAS# 550-43-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 550-43-6 | SDF | Download SDF |
| PubChem ID | 442939 | Appearance | Powder |
| Formula | C14H22N2O | M.Wt | 234.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
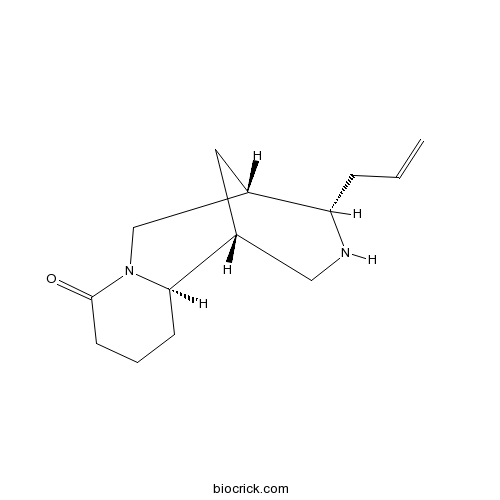
| SMILES | C=CCC1C2CC(CN1)C3CCCC(=O)N3C2 | ||
| Standard InChIKey | VTIPIBIDDZPDAV-MROQNXINSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Angustifoline has activity against gram-positive bacteria. |
| Targets | Antifection |

Angustifoline Dilution Calculator

Angustifoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.268 mL | 21.3402 mL | 42.6803 mL | 85.3606 mL | 106.7008 mL |
| 5 mM | 0.8536 mL | 4.268 mL | 8.5361 mL | 17.0721 mL | 21.3402 mL |
| 10 mM | 0.4268 mL | 2.134 mL | 4.268 mL | 8.5361 mL | 10.6701 mL |
| 50 mM | 0.0854 mL | 0.4268 mL | 0.8536 mL | 1.7072 mL | 2.134 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4268 mL | 0.8536 mL | 1.067 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- Busulfan
Catalog No.:BCC3742
CAS No.:55-98-1
- Hexamethonium Bromide
Catalog No.:BCC4561
CAS No.:55-97-0
- 1-Phenylbiguanide hydrochloride
Catalog No.:BCC6870
CAS No.:55-57-2
- Atropine sulfate
Catalog No.:BCN2716
CAS No.:55-48-1
- McN-A 343
Catalog No.:BCC7042
CAS No.:55-45-8
- Epinephrine HCl
Catalog No.:BCC4319
CAS No.:55-31-2
- Benzamide
Catalog No.:BCN5737
CAS No.:55-21-0
- HYOSCINE HYDROCHLORIDE
Catalog No.:BCN8331
CAS No.:55-16-3
- 3,3'',5-Triiodo-L-thyronine Sodium Salt
Catalog No.:BCN1419
CAS No.:55-06-1
- Shikonin acetyl
Catalog No.:BCN2452
CAS No.:54984-93-9
- Physalin D
Catalog No.:BCN7919
CAS No.:54980-22-2
- trans-Triprolidine hydrochloride
Catalog No.:BCC6742
CAS No.:550-70-9
- Afrormosine
Catalog No.:BCN3312
CAS No.:550-79-8
- Lupanine
Catalog No.:BCN5736
CAS No.:550-90-3
- Naphazoline HCl
Catalog No.:BCC4331
CAS No.:550-99-2
- NAADP tetrasodium salt
Catalog No.:BCC7808
CAS No.:5502-96-5
- (±)-Cloprostenol sodium salt
Catalog No.:BCC7315
CAS No.:55028-72-3
- Isorhamnetin-3-O-neohespeidoside
Catalog No.:BCN1234
CAS No.:55033-90-4
- Dammar-20(21)-en-3,24,25-triol
Catalog No.:BCN5734
CAS No.:55050-69-6
- 2,6,4'-Trihydroxy-4-methoxybenzophenone
Catalog No.:BCN7588
CAS No.:55051-85-9
- Protodioscin
Catalog No.:BCN6274
CAS No.:55056-80-9
- Diversoside
Catalog No.:BCN7537
CAS No.:55062-36-7
- Acitretin
Catalog No.:BCC1189
CAS No.:55079-83-9
Angustifoline inhibits human colon cancer cell growth by inducing autophagy along with mitochondrial-mediated apoptosis, suppression of cell invasion and migration and stimulating G2/M cell cycle arrest.[Pubmed:30941961]
J BUON. 2019 Jan-Feb;24(1):130-135.
PURPOSE: The prime objective of the present study was to investigate the anticancer properties of Angustifoline against COLO-205 human colon cancer cells. Its effects on cell autophagy, apoptosis, cell invasion and cell migration, and cell cycle arrest were also evaluated in the current study. METHODS: WST-1 assay was used to study cytotoxic effects of the compound on the cell viability. Effects on apoptosis and cell cycle arrest were evaluated by flow cytometry. In vitro wound healing assay and matrigel assay were carried out to study the effects of Angustifoline on cell migration and cell invasion respectively. To confirm autophagy, we evaluated the expression of several autophagy-associated proteins using Western blot assay along with transmission electron microscopy (TEM). RESULTS: The findings indicated that Angustifoline induced dose- and time-dependent cytotoxicity in COLO-205 human colon cancer cells along with inhibiting cancer cell colony formation. Angustifoline-treated cells exhibited cell shrinkage along with distortion of the normal cell morphology. Angustifoline-treated cells were also arrested in the G2/M phase of the cell cycle, showing strong dose-dependence. The compound also led to inhibition of cell migration and cell invasion. The results showed that treatment of these cells led to generation of autophagic cell vesicles. Furthermore, it was observed that the expression of Beclin-1 and LC3-II proteins was significantly upregulated in the Angustifoline-administered COLO-205 cells. CONCLUSIONS: In brief, the present study hints towards the potent anticancer potential of the natural product Angustifoline against COLO-205 human colon cancer cells with in depth mechanistic studies.


