BenzamideCAS# 55-21-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55-21-0 | SDF | Download SDF |
| PubChem ID | 2331 | Appearance | Powder |
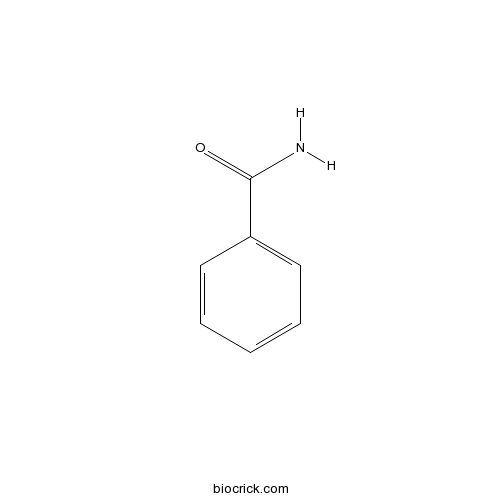
| Formula | C7H7NO | M.Wt | 121.1 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | >83.3mg/mL in DMSO | ||
| Chemical Name | benzamide | ||
| SMILES | C1=CC=C(C=C1)C(=O)N | ||
| Standard InChIKey | KXDAEFPNCMNJSK-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | PARP | Dopamine Receptor |

Benzamide Dilution Calculator

Benzamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.2576 mL | 41.2882 mL | 82.5764 mL | 165.1528 mL | 206.441 mL |
| 5 mM | 1.6515 mL | 8.2576 mL | 16.5153 mL | 33.0306 mL | 41.2882 mL |
| 10 mM | 0.8258 mL | 4.1288 mL | 8.2576 mL | 16.5153 mL | 20.6441 mL |
| 50 mM | 0.1652 mL | 0.8258 mL | 1.6515 mL | 3.3031 mL | 4.1288 mL |
| 100 mM | 0.0826 mL | 0.4129 mL | 0.8258 mL | 1.6515 mL | 2.0644 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- HYOSCINE HYDROCHLORIDE
Catalog No.:BCN8331
CAS No.:55-16-3
- 3,3'',5-Triiodo-L-thyronine Sodium Salt
Catalog No.:BCN1419
CAS No.:55-06-1
- Shikonin acetyl
Catalog No.:BCN2452
CAS No.:54984-93-9
- Physalin D
Catalog No.:BCN7919
CAS No.:54980-22-2
- Tamoxifen Citrate
Catalog No.:BCC4382
CAS No.:54965-24-1
- Albendazole
Catalog No.:BCC3718
CAS No.:54965-21-8
- Florilenalin
Catalog No.:BCN6422
CAS No.:54964-49-7
- 2-Oxopomolic acid
Catalog No.:BCN5732
CAS No.:54963-52-9
- Shikalkin
Catalog No.:BCC8359
CAS No.:54952-43-1
- Phytolaccagenic acid
Catalog No.:BCN8090
CAS No.:54928-05-1
- beta-Yohimbine
Catalog No.:BCN5733
CAS No.:549-84-8
- Quercetin 3-O-beta-D-xylopyranoside
Catalog No.:BCN2851
CAS No.:549-32-6
- Epinephrine HCl
Catalog No.:BCC4319
CAS No.:55-31-2
- McN-A 343
Catalog No.:BCC7042
CAS No.:55-45-8
- Atropine sulfate
Catalog No.:BCN2716
CAS No.:55-48-1
- 1-Phenylbiguanide hydrochloride
Catalog No.:BCC6870
CAS No.:55-57-2
- Hexamethonium Bromide
Catalog No.:BCC4561
CAS No.:55-97-0
- Busulfan
Catalog No.:BCC3742
CAS No.:55-98-1
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- Angustifoline
Catalog No.:BCN3205
CAS No.:550-43-6
- trans-Triprolidine hydrochloride
Catalog No.:BCC6742
CAS No.:550-70-9
- Afrormosine
Catalog No.:BCN3312
CAS No.:550-79-8
- Lupanine
Catalog No.:BCN5736
CAS No.:550-90-3
- Naphazoline HCl
Catalog No.:BCC4331
CAS No.:550-99-2
Density functional theory study of Rh(III)-catalyzed C-H activations and intermolecular annulations between benzamide derivatives and allenes.[Pubmed:25856513]
Inorg Chem. 2015 Apr 20;54(8):3958-69.
Density functional theory has been applied to gain insight into the Cp*Rh(OAc)2-catalyzed C-H activation and intermolecular annulation of Benzamide derivatives with allenes. The study shows that the reactions proceed in three steps: (1) C-H activation induced by Rh catalyst reacting with Benzamide derivatives, (2) carborhodation of allene, and (3) regeneration of Rh catalyst. The results indicate that the N-H deprotonation makes the following C-H activation much easier. The regio- and stereoselectivities of 1a (N-pivaloyloxy Benzamide)/2a (cyclohexylallene) and 1b (N-pivaloyloxy-4-methyl-Benzamide)/2b (1,1-dimethyl allene) depend on the allene carborhodation step. The steric hindrance effect is the dominant factor. We also discuss the reaction mechanism of 1c (N-methoxy Benzamide)/2a. The chemoselectivity between 1c/2a is determined by the N-O cleavage step. Replacement of OPiv by OMe leads to loss of the stabilization effect provided by C=O in OPiv. Additionally, Cp*Rh(OAc)(OPiv) is produced in the Cp*Rh(OAc)2 regeneration step, which can work as catalyst as well.


