Shikonin acetylCAS# 54984-93-9 |
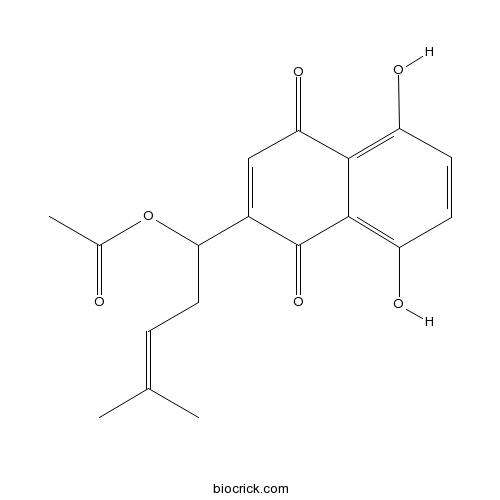
- Acetylshikonin
Catalog No.:BCN2665
CAS No.:24502-78-1
- Acetylalkannin
Catalog No.:BCX1034
CAS No.:34232-27-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 54984-93-9 | SDF | Download SDF |
| PubChem ID | 32464 | Appearance | Powder |
| Formula | C18H18O6 | M.Wt | 330.33 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [1-(5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl] acetate | ||
| SMILES | CC(=CCC(C1=CC(=O)C2=C(C=CC(=C2C1=O)O)O)OC(=O)C)C | ||
| Standard InChIKey | WNFXUXZJJKTDOZ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Acetylshikonin can effectively inhibit tumor cells, it can be used to treat hepatocellular carcinoma cells expressing hepatitis B virus X protein (HBX) by inducing ER stress , an oncoprotein from hepatitis B virus. Acetylshikonin inhibits the production of eicosanoid, is due to the attenuation of cytosolic phospholipase A(2) membrane recruitment via the decrease in [Ca(2+)](i) and to the blockade of cyclooxygenase and 5-lipoxygenase activity. |
| Targets | JNK | AChR | Bcl-2/Bax | ERK | MAPK | HO-1 | COX | Calcium Channel | HBVX |
| In vitro | Acetylshikonin induces apoptosis of hepatitis B virus X protein-expressing human hepatocellular carcinoma cells via endoplasmic reticulum stress.[Pubmed: 24769509]Eur J Pharmacol. 2014 Jul 15;735:132-40.
Acetylshikonin, a Novel AChE Inhibitor, Inhibits Apoptosis via Upregulation of Heme Oxygenase-1 Expression in SH-SY5Y Cells.[Pubmed: 24302971]Evid Based Complement Alternat Med. 2013;2013:937370.Acetylcholinesterase inhibitors are prominent alternative in current clinical treatment for AD patients. Therefore, there is a continued need to search for novel AChEIs with good clinical efficacy and less side effects. |
| Kinase Assay | The influence of acetylshikonin, a natural naphthoquinone, on the production of leukotriene B4 and thromboxane A2 in rat neutrophils.[Pubmed: 19232341]Eur J Pharmacol. 2009 Apr 1;607(1-3):234-43.
|
| Cell Research | Inhibitory effect of acetylshikonin on human gastric carcinoma cell line SGC-7901 in vitro and in vivo.[Pubmed: 19370777]World J Gastroenterol. 2009 Apr 21;15(15):1816-20.To investigate the inhibitory effect of Acetylshikonin on human gastric carcinoma cell line SGC-7901 and its mechanism.
|
| Structure Identification | AAPS PharmSciTech. 2014 Apr;15(2):425-33.Encapsulation of acetylshikonin by polyamidoamine dendrimers for preparing prominent nanoparticles.[Pubmed: 24449188]Acetylshikonin (AS) has demonstrated antitumor potential. However, the development of therapeutic applications utilizing AS is inhibited by its poor solubility in water. |

Shikonin acetyl Dilution Calculator

Shikonin acetyl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0273 mL | 15.1364 mL | 30.2728 mL | 60.5455 mL | 75.6819 mL |
| 5 mM | 0.6055 mL | 3.0273 mL | 6.0546 mL | 12.1091 mL | 15.1364 mL |
| 10 mM | 0.3027 mL | 1.5136 mL | 3.0273 mL | 6.0546 mL | 7.5682 mL |
| 50 mM | 0.0605 mL | 0.3027 mL | 0.6055 mL | 1.2109 mL | 1.5136 mL |
| 100 mM | 0.0303 mL | 0.1514 mL | 0.3027 mL | 0.6055 mL | 0.7568 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Physalin D
Catalog No.:BCN7919
CAS No.:54980-22-2
- Tamoxifen Citrate
Catalog No.:BCC4382
CAS No.:54965-24-1
- Albendazole
Catalog No.:BCC3718
CAS No.:54965-21-8
- Florilenalin
Catalog No.:BCN6422
CAS No.:54964-49-7
- 2-Oxopomolic acid
Catalog No.:BCN5732
CAS No.:54963-52-9
- Shikalkin
Catalog No.:BCC8359
CAS No.:54952-43-1
- Phytolaccagenic acid
Catalog No.:BCN8090
CAS No.:54928-05-1
- beta-Yohimbine
Catalog No.:BCN5733
CAS No.:549-84-8
- Quercetin 3-O-beta-D-xylopyranoside
Catalog No.:BCN2851
CAS No.:549-32-6
- 8-Oxyberberine
Catalog No.:BCN3135
CAS No.:549-21-3
- Amitriptyline HCl
Catalog No.:BCC5033
CAS No.:549-18-8
- Arborinine
Catalog No.:BCN7438
CAS No.:5489-57-6
- 3,3'',5-Triiodo-L-thyronine Sodium Salt
Catalog No.:BCN1419
CAS No.:55-06-1
- HYOSCINE HYDROCHLORIDE
Catalog No.:BCN8331
CAS No.:55-16-3
- Benzamide
Catalog No.:BCN5737
CAS No.:55-21-0
- Epinephrine HCl
Catalog No.:BCC4319
CAS No.:55-31-2
- McN-A 343
Catalog No.:BCC7042
CAS No.:55-45-8
- Atropine sulfate
Catalog No.:BCN2716
CAS No.:55-48-1
- 1-Phenylbiguanide hydrochloride
Catalog No.:BCC6870
CAS No.:55-57-2
- Hexamethonium Bromide
Catalog No.:BCC4561
CAS No.:55-97-0
- Busulfan
Catalog No.:BCC3742
CAS No.:55-98-1
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- Angustifoline
Catalog No.:BCN3205
CAS No.:550-43-6
- trans-Triprolidine hydrochloride
Catalog No.:BCC6742
CAS No.:550-70-9
The influence of acetylshikonin, a natural naphthoquinone, on the production of leukotriene B4 and thromboxane A2 in rat neutrophils.[Pubmed:19232341]
Eur J Pharmacol. 2009 Apr 1;607(1-3):234-43.
Both A23187 and formyl-Met-Leu-Phe (fMLP) induced the release of arachidonic acid and the production of thromboxane B(2) and leukotriene B(4) from rat neutrophils that were inhibited by acetylshikonin in a concentration-dependent manner. Acetylshikonin blocked exogenous arachidonic acid-induced leukotriene B(4) and thromboxane B(2) production in neutrophils and inhibited the enzymatic activity of ram seminal vesicles cyclooxygenase and human recombinant 5-lipoxygenase, whereas it had no effect on cytosolic phospholipase A(2) activity, in cell-free systems. 3-Morpholinosydnonimine- and 13S-hydroperoxy-9Z,11E-octadecadienoic acid (13-HpODE)-mediated dihydrorhodamine 123 oxidation (to assess the lipid peroxide and peroxynitrite scavenging activity) was reduced by acetylshikonin. The membrane recruitment of cytosolic phospholipase A(2) was inhibited, but the phosphorylation of cytosolic phospholipase A(2) was enhanced, by acetylshikonin in the A23187-induced response. Acetylshikonin alone stimulated extracellular signal regulated kinase (ERK) phosphorylation and enhanced this response in cells stimulated with A23187 and fMLP. The phosphorylation of ERKs and cytosolic phospholipase A(2) was attenuated by U0126, a mitogen-activated protein kinase (MAPK)/ERK kinase (MEK) inhibitor. Acetylshikonin facilitated both A23187- and fMLP-mediated translocation of 5-lipoxygenase to the membrane. Acetylshikonin attenuated both fMLP- and ionomycin-mediated [Ca(2+)](i) elevation. These results indicate that the inhibition of eicosanoid production by acetylshikonin is due to the attenuation of cytosolic phospholipase A(2) membrane recruitment via the decrease in [Ca(2+)](i) and to the blockade of cyclooxygenase and 5-lipoxygenase activity.
Inhibitory effect of acetylshikonin on human gastric carcinoma cell line SGC-7901 in vitro and in vivo.[Pubmed:19370777]
World J Gastroenterol. 2009 Apr 21;15(15):1816-20.
AIM: To investigate the inhibitory effect of acetylshikonin on human gastric carcinoma cell line SGC-7901 and its mechanism. METHODS: MTT assay was used to assess the inhibitory effect of acetylshikonin on proliferation of SGC-7901 cells. Apoptosis-inducing effect was determined by flow cytometry and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling with Hoechst staining. Expression of mRNA and protein in Bcl-2 and Bax was analyzed by reverse transcription-polymerase chain reaction and Western blot. Antitumor effect of acetylshikonin on a mouse SGC-7901 model was also determined. RESULTS: Forty-eight hours after treatment with acetylshikonin, MTT assay showed that acetylshikonin inhibited the proliferation of SGC-7901 cells in a dose-dependent manner. The half maximal inhibitory concentration of acetylshikonin to SGC-7901 cells was 0.428 +/- 0.07 mg/L. Cell shrinkage, nuclear pyknosis and chromatin condensation, which are the characteristics of cell apoptosis, were observed in treated SGC-7901 cells and the percentage of apoptosis increased in a dose-dependent manner. Acetylshikonin down-regulated the expression of Bcl-2 and up-regulated the expression of Bax in the treated SGC-7901 cells compared with the controls. The experiment in vivo showed that 0.5, 1, and 2 mg/kg of acetylshikonin significantly inhibited the growth of tumor in the mouse SGC-7901 model, with an inhibitory rate of 25.00%-55.76%. CONCLUSION: Acetylshikonin inhibits the growth of SGC-7901 cells in vitro and in vivo by inducing cell apoptosis.
Encapsulation of acetylshikonin by polyamidoamine dendrimers for preparing prominent nanoparticles.[Pubmed:24449188]
AAPS PharmSciTech. 2014 Apr;15(2):425-33.
Acetylshikonin (AS) has demonstrated antitumor potential. However, the development of therapeutic applications utilizing AS is inhibited by its poor solubility in water. In the present work, polyamidoamine (PAMAM) dendrimers and their PEGylated derivatives were employed to increase the solubility of AS. A distinct color transition was observed during the encapsulation of AS suggesting strong intermolecular forces between PAMAM and AS. Ultraviolet-visible, high-performance liquid chromatography, and (1)H NMR were used to verify the interaction between PAMAM and AS. The maximum amount of combined AS to each PAMAM molecule was determined. The cytotoxicity of AS nanoparticles was evaluated against leukemia (K562) and breast cancer (SK-BR-3) cell lines; the AS nanoparticles were shown to effectively inhibit tumor cells.
Acetylshikonin, a Novel AChE Inhibitor, Inhibits Apoptosis via Upregulation of Heme Oxygenase-1 Expression in SH-SY5Y Cells.[Pubmed:24302971]
Evid Based Complement Alternat Med. 2013;2013:937370.
Acetylcholinesterase inhibitors are prominent alternative in current clinical treatment for AD patients. Therefore, there is a continued need to search for novel AChEIs with good clinical efficacy and less side effects. By using our in-house natural product database and AutoDock Vina as a tool in docking study, we have identified twelve phytochemicals (emodin, aloe-emodin, chrysophanol, and rhein in Rhei Radix Et Rhizoma; xanthotoxin, phellopterin, alloisoimperatorin, and imperatorin in Angelicae dahuricae Radix; shikonin, acetylshikonin, isovalerylshikonin, and beta,beta-dimethylacrylshikonin in Arnebiae Radix) as candidates of AChEIs that were not previously reported in the literature. In addition to AChEI activity, a series of cell-based experiments were conducted for the investigation of their neuroprotective activities. We found that acetylshikonin and its derivatives prevented apoptotic cell death induced by hydrogen peroxide in human and rat neuronal SH-SY5Y and PC12 cells at 10 muM. We showed that acetylshikonin exhibited the most potent antiapoptosis activity through the inhibition of the generation of reactive oxygen species as well as protection of the loss of mitochondria membrane potential. Furthermore, we identified for the first time that the upregulation of heme oxygenase 1 by acetylshikonin is a key step mediating its antiapoptotic activity from oxidative stress in SH-SY5Y cells.
Acetylshikonin induces apoptosis of hepatitis B virus X protein-expressing human hepatocellular carcinoma cells via endoplasmic reticulum stress.[Pubmed:24769509]
Eur J Pharmacol. 2014 Jul 15;735:132-40.
Since it has been known that shikonin derived from a medicinal plant possesses anti-cancer activity, we wonder whether acetylshikonin (ASK), a derivate of shikonin, can be used to treat hepatocellular carcinoma cells expressing hepatitis B virus X protein (HBX), an oncoprotein from hepatitis B virus. When ASK was added to Hep3B cells stably expressing HBX, it induced apoptosis in a dose-dependent manner. ASK induced upregulation and export of Nur77 to the cytoplasm and activation of JNK. Likewise, suppression of Nur77 and JNK inactivation protected the cells from ASK-induced apoptosis, indicating that Nur77 upregulation and JNK activation were required for ASK-mediated apoptosis. Furthermore, ASK increased the expression of Bip and ubiquitination levels of cellular proteins, features of endoplasmic reticulum (ER) stress, via the production of reactive oxygen species in a dose-dependent manner. Suppression of reactive oxygen species with N-acetylcysteine reduced levels of Bip protein and ubiquitination levels of cellular proteins during ASK treatment, leading to protection of cells from apoptosis. Cycloheximide treatment reduced ASK-induced ER stress, suggesting that protein synthesis is involved in ASK-induced ER stress. Moreover, we showed using salubrinal, an ER stress inhibitor that reactive oxygen species production, JNK activation, and Nur77 upregulation and its translocation to cytoplasm are necessary for ER-induced stress. Interestingly, we found that JNK inactivation suppresses ASK-induced ER stress, whereas Nur77 siRNA treatment does not, indicating that JNK is required for ASK-induced ER stress. Accordingly, we report that ASK induces ER stress, which is prerequisite for apoptosis of HBX-expressing hepatocellular carcinoma cells.


